Oceanologia No. 67 (4) / 25
Original Research Articles
-
Impact of climate change on the coastal water
temperature of lagoons in the southern Baltic Sea
in the period 1951–2020: Barbara Pius
-
Mutual influence of wind-driven flow and the wave bed boundary layer in the remote foreshore of a non-tidal sea: Magdalena Stella-Bogusz, Rafał Ostrowski, Grzegorz R. Cerkowniak
-
Diel patterns of fish activity in the Vistula Lagoon’s
littoral zone: integrating acoustic and net fishing
methods: Beata Schmidt, Ryszard Kornijów
-
Dynamics of ice phenomena on the lake shores based on ice scars method – study from the Southern Baltic Sea coast: Józef P. Girjatowicz, Tomasz A. Łabuz, Małgorzata Świątek
-
Decadal change of benthic macroinvertebrates driven by multiple stresses in the Changjiang Estuary in summer: Yanbin Tang, Bin Wang, Dewang Li, Xiao Ma, Zhinbing Jian, Yibo Liao, Qinghe Liu, Rongliang Zhang, Jiangning Zeng, Jianfang Chen, Chenghua Li, Lu Shou
-
Long-term variability of sound speed conditions in Hornsund fjord, Svalbard, between 2001 and 2019: Pavani Vithana Madugeta Vidanamesthrige, Natalia Gorska, Oskar Głowacki
-
Perspectives of seasonal hydrography and water masses in Saudi waters of the Arabian Gulf: Mohamed Asharaf, V.M. Aboobacker, C.P. Abdulla, Thadickal V. Joydas, Karuppasamy P. Manikandan, M. Rafeeq, Abdulaziz Al-Suwailem, P. Vethamony
-
Statistical downscaling of global climate projections over the Egyptian Red Sea coast: Mohamed Shaltout, Ahmed Abdelhamid, Ahmed Adel, Mohamed Gad, Mohamed Elbessa
-
Benthic diatom communities in deeper areas of the German Baltic Sea: Marjan Janßen, Israel Barrantes, Mirko Dressler, Karin Glaser, Ulf Karsten
-
First record of Moerisia cf. inkermanica Paltschikowa-Ostroumowa, 1925 (Hydrozoa, Moerisiidae) in the Gulf of Gdańsk (southern Baltic Sea): Michał Olenycz, Marcin Kalarus
-
Does mesh size matter? Influence of mesh size on estimation of meiofauna abundance, biomass and on size spectra: Barbara Górska, Katarzyna Grzelak, Bodil A. Bluhm, Silvia Hess, Maria Włodarska-Kowalczuk
Original Research Articles
Impact of climate change on the coastal water temperature of lagoons in the southern Baltic Sea in the period 1951–2020
Oceanologia, 67 (4)/2025, 67401, 18 pp.
https://doi.org/10.5697/WECV1208
Bożena Pius
Faculty of Earth Sciences and Spatial Management, Department of Hydrology, Cryology and Water Management, Nicolaus Copernicus University in Toruń, Lwowska 1, 87-100, Toruń, Poland;
e-mail: bpius@umk.pl (B. Pius)
Keywords:
Lagoons of the southern Baltic Sea; Water surface temperature (WST); Climate warming; Trend analysis; Warming of the lagoons
Received: 28 October 2024; revised: 19 August 2025; accepted: 16 September 2025
Highlights
- Long-term water temperature fluctuations in southern Baltic lagoons analysed over 70 years (1951–2020).
- Unique in situ data collected manually over decades underpin the analysis.
- Dynamic and region-specific warming trends identified in the Vistula and Szczecin Lagoons.
- Seasonal warming patterns are most pronounced in spring and winter periods.
- Robust statistical methods, including the Mann-Kendall test, ensure the reliability of results.
- Findings provide insights for conservation strategies in sensitive coastal ecosystems.
Abstract
The study examines long-term trends in water surface temperature (WST) in the Vistula and Szczecin Lagoons within the southern Baltic Sea from 1951 to 2020. Based on in situ data, temperature variability was assessed using both parametric linear regression and the non-parametric Mann-Kendall test. The results reveal a statistically significant increase in water temperatures, particularly during spring and winter. The average warming rate reached 0.23°C and 0.26°C per decade in the Vistula and Szczecin Lagoons, respectively. A strong correlation (r𝑟 = 0.60–0.93) was observed between air and surface water temperature. Extreme temperature events are becoming more frequent, with the lowest percentile values rising markedly over time. Winter temperatures exceeding 3°C are now common, and ice cover has diminished or disappeared. These trends highlight the regional impacts of climate change on coastal lagoon ecosystems and their seasonal dynamics. The findings provide valuable insights for future monitoring and management of vulnerable brackish water environments.
References 
Al-Shehhi, M. R., 2022.
Uncertainty in satellite sea surface temperature
with respect to air temperature, dust level, wind speed and solar
position, Reg. Stud. Mar. Sci. 53, 102385.
https://doi.org/10.1016/j.rsma.2022.102385
Amos, C.L., Umgiesser, G., Ghezzo, M., Kassem, H., Ferrarin, C., 2017.
Sea
surface temperature trends in Venice Lagoon and the adjacent waters. J.
Coast. Res. 33(2), 385-395.
https://doi.org/10.2112/JCOASTRES-D-16-00017.1
BACC I., Team A. (Eds.), 2015.
Second assessment of climate change for the
Baltic Sea basin, Springer Open.
Bertolini, C. and Pastres, R., 2021.
Tolerance landscapes can be used to
predict species-specific responses to climate change beyond the marine heatwave
concept: Using tolerance landscape models for an ecologically meaningful
classification of extreme climate events. Estuar. Coast. Shelf Sci. 252,
p.107284.
https://doi.org/10.1016/j.ecss.2021.107284
Bamber, J. L., Oppenheimer, M., Kopp, R. E., et al., 2019.
Ice sheet
contributions to future sea-level rise from structured expert judgment.
Proc. Nat. Acad. Sci. 116(23), 11195-11200.
https://doi.org/10.1073/pnas.1817205116
Brennan, C. E., Blanchard, H., Fennel, K., 2016.
Putting temperature and
oxygen thresholds of marine animals in context of environmental change: a
regional perspective for the Scotian Shelf and Gulf of St. Lawrence, PLoS
One 11(12), e0167411.
https://doi.org/10.1371/journal.pone.0167411
Brierley, A. S., Kingsford, M. J., 2009.
Impacts of climate change on
marine organisms and ecosystems, Curr. Biol. 19(14), R602-R614.
https://doi.org/10.1016/j.cub.2009.05.046
Cai, R. S., Tan, H. J., Kontoyiannis, H., 2017.
Robust surface warming in
offshore China seas and its relationship to the East Asian Monsoon wind field
and ocean forcing on inter-decadal time scales, J. Clim. 30(22),
8987-9005.
https://doi.org/10.1175/JCLI-D-16-0016.1
Calvo, E., SimoÌ, R., Coma, R., et al., 2011.
Effects of climate change
on Mediterranean marine ecosystems: the case of the Catalan Sea, Clim.
Res. 50(1), 1-29.
https://doi.org/10.3354/cr01040
Cieśliński, R., Chlost, I., Szydłowski, M., 2024
. Impact of new,
navigable canal through the Vistula Spit on the hydrologic balance of the
Vistula Lagoon (Baltic Sea), J. Mar. Syst. 241, 103908.
https://doi.org/10.1016/j.jmarsys.2023.103908
Curiel, D., Rismondo, A., Bellemo, G., et al., 2004.
Macroalgal biomass and
species variations in the Lagoon of Venice (Northern Adriatic Sea, Italy):
1981-1998, Sci. Mar. 68(1), 57-67.
https://doi.org/10.3989/scimar.2004.68n157
Čerkasova, N., Mėžinė, J., Idzelytė, R., et al., 20
24. Exploring
variability in climate change projections on the Nemunas River and Curonian
Lagoon: coupled SWAT and SHYFEM modeling approach, Ocean Sci. 20(5),
1123-1147.
https://doi.org/10.5194/os-20-1123-2024
Dailidienė, I., Baudler, H., Chubarenko, B., Navrotskaya, S., 2011.
Long
term water level and surface temperature changes in the lagoons of the southern
and eastern Baltic, Oceanologia 53(1-TI), 293-308.
https://doi.org/10.5697/oc.53-1-TI.293
Dailidienė, I., Davuliene, L., 2008.
Salinity trend and variation in the
Baltic Sea near the Lithuanian coast and in the Curonian Lagoon in
1984-2005. J. Marine Syst. 74, S20-S29.
https://doi.org/10.1016/j.jmarsys.2008.01.014
Dutheil, C., Meier, H. E. M., Gröger, M., et al., 2022.
Understanding
past and future sea surface temperature trends in the Baltic Sea, Clim.
Dynam. 58(11), 3021-3039.
https://doi.org/10.1007/s00382-021-06084-1
Fernández-Nóvoa, D., Costoya, X., DeCastro, M., et al., 2021
. Influence
of the mightiest rivers worldwide on coastal sea surface temperature
warming, Sci. Total Environ. 768, 144915.
https://doi.org/10.1016/j.scitotenv.2020.144915
Gaertner-Mazouni, N., De Wit, R., 2012.
Exploring new issues for coastal
lagoons monitoring and management, Estuar. Coast. Shelf Sci. 114, 1-6.
https://doi.org/10.1016/j.ecss.2012.07.008
Galbraith, P. S., Larouche, P., 2013.
Trends and variability in air and sea
surface temperatures in eastern Canada, [in:] Aspects of Climate Change in
the Northwest Atlantic off Canada, Can. Tech. Rep. Fish. Aquat. Sci. 3045,
1-18.
Garbrecht, J., Fernandez, G. P., 1994.
Visualization of trends and
fluctuations in climatic records, Water Resour. Bull. 30(2), 297-306.
https://doi.org/10.1111/j.1752-1688.1994.tb03292.x
Goebeler, N., Norkko, A., Norkko, J., 2022.
Ninety years of coastal
monitoring reveals baseline and extreme ocean temperatures are increasing off
the Finnish coast, Commun. Earth Environ. 3, 215.
https://doi.org/10.1038/s43247-022-00545-z
Goikoetxea, N., Borja, Á., Fontán, A., et al., 2009
. Trends and
anomalies in sea-surface temperature, observed over the last 60 years, within
the southeastern Bay of Biscay, Cont
. Shelf Res. 29(8),
1060-1069.
https://doi.org/10.1016/j.csr.2008.11.014
Graf, R., Vyshnevskyi, V., 2023.
Thermal regime of the Vistula River mouth
and the Gdańsk Bay, Geogr. Pol. 96(4), 459-471.
https://doi.org/10.7163/GPol.0264
Graf, R., WrzesinÌski, D., 2020. D
etecting patterns of changes in river
water temperature in Poland, Water 12(5), 1442.
https://doi.org/10.3390/w12051327
Grebmeier, J. M., 2012.
Shifting patterns of life in the Pacific Arctic and
sub-Arctic seas, Annu. Rev. Mar. Sci. 4, 63-78.
https://doi.org/10.1146/annurev-marine-120710-100926
Gröger, J. P., Hinrichsen, H.-H., Polte, P., 2014.
Broad-scale climate
influences on spring-spawning herring (Clupea harengus L.) recruitment in the
Western Baltic Sea, PLoS One 9, e87525.
https://doi.org/10.1371/journal.pone.0087525
Haase, P., Bowler, D. E., Baker, N. J., et al., 2023.
The recovery of
European freshwater biodiversity has come to a halt, Nature 620(7974),
582-588. h
ttps://doi.org/10.1038/s41586-023-06400-1
Hannah, D. M., Garner, G., 2015.
River water temperature in the United
Kingdom: changes over the 20th century and possible changes over the 21st
century, Prog. Phys. Geogr. 39(1), 68-92.
https://doi.org/10.1177/0309133314550669
Hansen, J., Sato, M., 2020.
Global warming acceleration, Earth Inst.,
Columbia Univ., 14 pp.
Harley, C. D., Hughes, A. R., Hultgren, K. M., et al., 2006.
The impacts of
climate change in coastal marine systems, Ecol. Lett. 9(2), 228-241.
https://doi.org/10.1111/j.1461-0248.2005.00871.x
Hoegh-Guldberg, O., Bruno, J. F., 2010.
The impact of climate change on the
world's marine ecosystems, Science 328(5985), 1523-1528.
https://doi.org/10.1126/science.1189930
Huang, B., Thorne, P., Smith, T., et al., 2015a.
Further exploring and
quantifying uncertainties for extended reconstructed sea surface temperature
(ERSST) version 4(v4), J. Climate 29, 3119-3142.
https://doi.org/10.1175/JCLI-D-15-0430.1
IPCC, 2021.
Climate Change 2021: The Physical Science Basis. Contribution
of Working Group I to the Sixth Assessment Report of the Intergovernmental
Panel on Climate Change [Masson-Delmotte, V., Zhai, P., Pirani, A., et al.
(eds.)], Cambridge Univ. Press, Cambridge, UK; New York, NY, USA.
https://doi.org/10.1017/9781009157896
IPCC, 2023.
Climate Change 2023: Synthesis Report. Contribution of Working
Groups I, II and III to the Sixth Assessment Report of the Intergovernmental
Panel on Climate Change [Core Writing Team, Lee, H., Romero, J. (eds.)],
IPCC, Geneva, Switzerland, 184 pp.
https://doi.org/10.59327/IPCC/AR6-9789291691647
Jane, S. F., Hansen, G. J. A., Kraemer, B. M. et al., 2021.
Widespread
deoxygenation of temperate lakes. Nature 594(7861), 66-70.
https://doi.org/10.1038/s41586-021-03550-y
Jaswal, A. K., Singh, V., Bhambak, S. R., 2012.
Relationship between sea
surface temperature and surface air temperature over Arabian Sea, Bay of Bengal
and Indian Ocean, J. Indian Geophys. Union 16(2), 41-53.
Kędra, M., Wiejaczka, Ł., 2018.
Climatic and dam-induced impacts on river
water temperature: assessment and management implications, Sci. Total
Environ. 626, 1474-1483.
https://doi.org/10.1016/j.scitotenv.2017.10.044
Kejna, M., Rudzki, M., 2021.
Spatial diversity of air temperature changes
in Poland in 1961-2018, Theor. Appl. Climatol. 143(3-4), 1361-1379.
https://doi.org/10.1007/s00704-020-03487-8
Kennedy, J. J., Rayner, N. A., Atkinson, C. P., 2019.
An ensemble data set
of sea-surface temperature change from 1850: the Met Office Hadley Centre
HadSST.4.0.0.0 data set, J. Geophys. Res. Atmos. 124(14), 7719-7763.
https://doi.org/10.1029/2018JD029867
Kniebusch, M., Meier, H. M., Neumann, T., Börgel, F., 2019.
Temperature
variability of the Baltic Sea since 1850 and attribution to atmospheric forcing
variables, J. Geophys. Res. Oceans 124(6), 4168-4187. h
ttps://doi.org/10.1029/2018JC013948
Kozlov, I., Dailidienė, I., Korosov, A., Klemas, V., Mingėlaitė, T.,
201
4. MODIS-based sea surface temperature of the Baltic Sea Curonian
Lagoon, J. Mar. Syst. 129, 157-165.
https://doi.org/10.1016/j.jmarsys.2012.05.011
Kundzewicz, Z. W., Matczak, P., 2012.
Climate change regional review:
Poland. WIREs Climate Change, 3(4), 297-311.
https://doi.org/10.1002/wcc.179
Kundzewicz, Z. W., Robson, A. J., 2004.
Change detection in hydrological
records — a review of the methodology. Hydrol. Sci. J. 49(1), 7-19.
https://doi.org/10.1623/hysj.49.1.7.53993
Laakso, L., Mikkonen, S., Drebs, A., et al., 2018.
100 years of atmospheric
and marine observations at the Finnish Utö Island in the Baltic Sea,
Ocean Sci. 14(4), 617-632.
https://doi.org/10.5194/os-14-617-2018
Lehmann, A., Myrberg, K., Post, P., Chubarenko, I., Dailidiene, I., Hinrichsen,
H.H., Hüssy, K., Liblik, T., Meier, H.M., Lips, U., Bukanova, T., 2022.
Salinity dynamics of the Baltic Sea. Earth Syst. Dynamk. 13(1),
373-392.
https://doi.org/10.5194/esd-13-373-2022
López Garcı́a, M. J., 2020
. SST Comparison of AVHRR and MODIS Time
Series in the Western Mediterranean Sea, Remote Sens. 12(14), 2241.
https://doi.org/10.3390/rs12142241
Łomniewski, K., 1958. Zalew Wiślany, PWN, Warszawa, 117 pp. Marszelewski, W.,
Pius, B., 2016.
Long-term changes in temperature of river waters in the
transitional zone of the temperate climate: a case study of Polish rivers,
Hydrol. Sci. J. 61(8), 1430-1442.
https://doi.org/10.1080/02626667.2015.1040800
Meier, H. M., Dieterich, C., Eilola, K., et al., 2019.
Future projections
of record-breaking sea surface temperature and cyanobacteria bloom events in
the Baltic Sea. Ambio 48, 1362-1376.
https://doi.org/10.1007/s13280-019-01235-5
Meier M., Kjellström E., Graham P., 2006.
Estimating uncertainties of
projected Baltic sea salinity in the late 21st century. Geophys. Res.
Lett. 33,
https://doi.org/10.1029/2006GL026488
Mohseni, O., Stefan, H. G., 1999.
Stream temperature/air temperature
relationship: a physical interpretation, J. Hydrol. 218(3-4), 128-141.
https://doi.org/10.1016/S0022-1694(99)00034-7
Morrill, J. C., Bales, R. C., Conklin, M. H., 2005.
Estimating stream
temperature from air temperature: implications for future water quality,
J. Environ. Eng. 131(1), 139-146.
https://doi.org/10.1061/(ASCE)0733-9372(2005)131:1(139)
Neumann, T., Eilola, K., Gustafsson, B., et al., 2012.
Extremes of
temperature, oxygen and blooms in the Baltic Sea in a changing climate,
Ambio 41, 574-585.
https://doi.org/10.1007/s13280-012-0321-2
O’carroll, A.G., Armstrong, E.M., Beggs, H.M., et al., 2019.
Observational needs of sea surface temperature. Front. Mar. Sci. 6, p.
420.
https://doi.org/10.3389/fmars.2019.00420
Occhipinti-Ambrogi, A., 2007.
Global change and marine communities: alien
species and climate change, Mar. Pollut. Bull. 55(7-9), 342-352.
https://doi.org/10.1016/j.marpolbul.2006.11.014
Oliver, E. C. J., Donat, M. G., Burrows, M. T., et al., 2018.
Longer and
more frequent marine heatwaves over the past century, Nat. Commun. 9,
1324.
https://doi.org/10.1038/s41467-018-03732-9
O’Reilly, C. M., Sharma, S., Gray, D. K., et al., 2015.
Rapid and highly
variable warming of lake surface waters around the globe, Geophys. Res.
Lett. 42(24), 10773-10781.
https://doi.org/10.1002/2015GL066235
Pérez-Ruzafa A., Marcos C., Pérez-Ruzafa I.M., Barcala E., Hegazi M.I.,
Quispe J., 2007
. Detecting changes resulting from human pressure in a
naturally quick-changing and heterogeneous environment: spatial and temporal
scales of variability in coastal lagoons. Estuar. Coast. Shelf Sci. 75,
175-188.
https://doi.org/10.1016/j.ecss.2007.04.030
Pérez-Ruzafa A., Marcos C., 2012.
Fisheries in coastal lagoons: An
assumed but poorly researched aspect of the ecology and functioning of coastal
lagoons. Estuar. Coast. Shelf Sci. 110, 15-31.
https://doi.org/10.1016/j.ecss.2012.05.025
Pilgrim J.M., Fang X., Stefan H.G., 1998.
Stream temperature correlations
with air temperatures in Minnesota: implications for climate warming. J.
Am. Water Resour. Assoc. 34(5), 1109-1121.
https://doi.org/10.1111/j.1752-1688.1998.tb04158.x
Polte P., Kotterba P., Hammer C., Gröhsler T., 2014.
Survival bottlenecks
in the early ontogenesis of Atlantic herring (Clupea harengus, L.) in coastal
lagoon spawning areas of the western Baltic Sea. ICES J. Mar. Sci. 71,
982-990.
https://doi.org/10.1093/icesjms/fst050
Ptak, M., Sojka, M., Nowak, B., 2019.
Characteristics of daily water
temperature fluctuations in Lake Kierskie (West Poland). Quaestiones
Geographicae 38(2), 97-107.
https://doi.org/10.2478/quageo-2019-0017
Ridgway K.R., Ling S.D., 2023.
Three decades of variability and warming of
nearshore waters around Tasmania. Prog. Oceanogr. 215, 102902.
https://doi.org/10.1016/j.pocean.2023.103046
Rijnsdorp A.D., Peck M.A., Engelhard G.H., Möllmann C., Pinnegar J.K., 2009.
Resolving the effect of climate change on fish populations. ICES J.
Mar. Sci. 66(7), 1570-1583.
https://doi.org/10.1093/icesjms/fsp056
Smit A.J., Roberts M., Anderson R.J., Dufois F., Dudley S.F., Bornman T.G.,
Olbers J., Bolton J.J., 2013.
A coastal seawater temperature dataset for
biogeographical studies: large biases between in situ and remotely-sensed data
sets around the coast of South Africa. PLoS One 8(12), e81944.
https://doi.org/10.1371/journal.pone.0081944
Smol, J. P., Wolfe, A. P., Birks, H. J. B., et al., 2005.
Climatedriven
regime shifts in the biological communities of arctic lakes. Proc. Nat.
Acad. Sci. 102(12), 4397-4402.
https://doi.org/10.1073/pnas.0500245102
Stockmayer, V., Lehmann, A., 2023.
Variations of temperature, salinity and
oxygen of the Baltic Sea for the period 1950 to 2020. Oceanologia 65(3),
466-483.
https://doi.org/10.1016/j.oceano.2023.02.002
Stramska M., Białogrodzka J., 2015.
Spatial and temporal variability of
sea surface temperature in the Baltic Sea based on 32 years (1982-2013) of
satellite data. Oceanologia 57(3), 223-235. h
ttps://doi.org/10.1016/j.oceano.2015.04.004
Şen Z., 2017.
Innovative trend methodologies in science and
engineering. Springer Int. Publ., New York, 349 pp.
https://doi.org/10.1007/978-3-319-52338-5
Van Vliet M.T.H., Ludwig F., Zwolsman J.J.G., Weedon G.P., Kabat P., 2011.
Global river temperatures and sensitivity to atmospheric warming and
changes in river flow. Water Resour. Res. 47(2), W02544.
https://doi.org/10.1029/2010WR009198
Van Wynsberge S., Menkes C., Le Gendre R., Passfield T., Andréfouët S.,
2017
. Are sea surface temperature satellite measurements reliable proxies
of lagoon temperature in the South Pacific? Estuar. Coast. Shelf Sci. 199,
117-124.
https://doi.org/10.1016/j.ecss.2017.09.033
Venegas-Cordero N., Kundzewicz Z.W., Jamro S., et. al., 2022.
Detection of
trends in observed river floods in Poland. J. Hydrol. Reg. Stud. 41,
101098.
https:
//doi.org/10.1016/j.ejrh.2022.101098
Wang J., Xu C., Hu M., Li Q., Yan Z., Jones P., 2018.
Global land surface
air temperature dynamics since 1880. Int. J. Climatol. 38(S1), e466-e474.
https://doi.org/10.1002/joc.5384
Webb B.W., Nobilis F., 2007.
Long-term changes in river temperature and the
influence of climatic and hydrological factors. Hydrol. Sci. J. 52(1),
74-85.
https://doi.org/10.1623/hysj.52.1.74
Wolf M.A., Sfriso A., Moro I., 2014.
Thermal pollution and settlement of
new tropical alien species: the case of Grateloupia yinggehaiensis (Rhodophyta)
in the Venice Lagoon. Estuar. Coast. Shelf Sci. 147, 11-16.
https://doi.org/10.1016/j.ecss.2014.05.020
Zalewska T., Wilman B., Łapeta B., Marosz M., Biernacik D., Wochna A., Iwaniak
M., 2023.
Seawater temperature changes in the southern Baltic Sea
(1959-2019) forced by climate change. Oceanologia 66(1), 37-55.
https://doi.org/10.1016/j.oceano.2023.08.001
Mutual influence of wind-driven flow and the wave bed boundary layer in the remote foreshore of a non-tidal sea
Oceanologia, 67 (4)/2025, 67402, 11 pp.
https://doi.org/10.5697/SUEC1393
Magdalena Stella-Bogusz1,*, Rafał Ostrowski1, Grzegorz R. Cerkowniak1,2
1Institute of Hydro-Engineering, Polish Academy of Sciences, ul. Kościerska 7, 80–328 Gdańsk, Poland;
e-mail: m.stella@ibwpan.gda.pl (M. Stella-Bogusz)
2University of Gdańsk, Marszałka Piłsudskiego 46, 81–378 Gdynia, Poland
*corresponding author
Keywords:
Modified logarithmic velocity distribution; Bed shear stresses; Wave and wave-current bed boundary layers; Wind-driven current
Received: 15 July 2024; revised: 8 September 2025; accepted: 15 September 2025
Highlights
- Modified wave- or wave-current bed boundary layer impact on the wind-induced current.
- Modified logarithmic profile describing the wind-driven flow velocity.
- Strong effect of the wave-induced nearbed turbulence on the wind-driven current.
- Negligible effect of wind-driven current on bed boundary layer.
Abstract
A new model of the wind-driven current is presented. The solution comprises the stationary flow and wave-driven nearbed oscillatory velocities. The wave-related bed boundary layer causes additional shear stresses that affect the wind-driven current. Both the wave boundary layer (WBL) and the wave–current boundary layer (WCBL) are considered. The bed boundary layer produces a modified logarithmic velocity distribution in the wind-driven current model. The results of modelled profiles are verified by measurement data of wind, wave and current characteristics. All measurements are conducted in the vicinity of the Coastal Research Station (CRS) in Lubiatowo, where wave and current data were collected approximately 2.8 km (1.5 NM) from the coastline, at depth ca. 17 m, whereas the wind parameters were measured on land near the Station. The investigation area hydrodynamics is typical of the south Baltic Sea coast. Reduction of flow velocities near the seabed, particularly distinct under the wave-dominated regime, is the main feature of the new model. The new modified logarithmic profile defining velocity vertical distributions shows good agreement with the measurements. Furthermore, it is confirmed that the wave-induced nearbed turbulence strongly affects the wind-driven current, while the wind-driven current has an insignificant influence on the bed boundary layer.
References 
Baas, J., Malarkey, J., Lichtman, I., Amoudry, L., Thorne, P., Hope, J.,
Peakall, J., Paterson, D., Bass, S., Cooke, R., Manning, A., Parsons, D., Ye,
L., 2021.
Current- and Wave-Generated Bedforms on Mixed Sand–Clay
Intertidal Flats: A New Bedform Phase Diagram and Implications for Bed
Roughness and Preservation Potential. Front. Earth Sci. 9.
https://doi.org/10.3389/feart.2021.747567
Brevik, I., 1981.
Oscillatory rough turbulent boundary layers. J.
Waterw. Port Coast. Ocean Div. 107 (3), 175–188.
https://doi.org/10.1061/JWPCDX.0000261
Cerkowniak, G.R., Ostrowski, R., Stella, M., 2015a.
Depth of closure in the
multi-bar non-tidal nearshore zone of the Baltic Sea: Lubiatowo (Poland) case
study. Bull. Maritime Inst. Gdańsk, 30 (1), 180–188.
http://doi.org/10.5604/12307424.1185577
Cerkowniak, G.R., Ostrowski, R., Stella, M., 2015b.
Wave-Induced Sediment
Motion Beyond the Surf Zone: Case Study of Lubiatowo (Poland). Arch.
Hydro-Eng. Environ. Mech. 62 (1–2), 27–39.
Cerkowniak, G.R., Ostrowski, R., Pruszak, Z., 2017.
Application of Dean’s
curve to the investigation of the long-term evolution of the southern Baltic
multi-bar shore profile. Oceanologia 59 (1), 18–27.
http://dx.doi.org/10.1016/j.oceano.2016.06.001
Chen, X., Hu, X., 2020.
Explicit approximation for velocity and sediment
flux above mobile sediment bed beneath current and asymmetric wave.
Coastal Eng. 157.
https://doi.org/10.1016/j.coastaleng.2020.103635
Dean, R.G., 2002.
Beach Nourishment. Theory and Practice. Advanced
Series on Ocean Engineering Vol. 18. World Sci. Publ. Co. Pte. Ltd., 399 pp.
https://doi.org/10.1142/2160
Egan, G., Cowherd, M., Fringer, O., Monismith, S., 2019.
Observations of
near-bed shear stress in a shallow, waveand current-driven flow. J.
Geophys. Res. 124, 6323–6344.
https://doi.org/10.1029/2019JC015165
Fredsøe, J., 1984.
Turbulent boundary layer in combined wave-current
motion. J. Hydraulic Eng. 110 (HY8), 1103–1120.
https://doi.org/10.1061/(ASCE)0733-9429(1984)110:8(1103)
Grant, W.D., Madsen, O.S., 1979.
Combined wave and current interaction with
a rough bottom. J. Geophys. Res. 84 (C4), 1797–1808.
https://doi.org/10.1029/JC084iC04p01797
Kemp, P., Simons, R., 1982.
The interaction between waves and a turbulent
current: Waves propagating with the current. J. Fluid Mech. 116,
227–250.
https://doi.org/10.1017/S0022112082000445
Kondo, J., Sato, T., 1982.
The Determination of the von Kármán
Constant. J. Meteorol. Soc. Japan, 60 (1), 461–471. Krauss, W., 2001.
Chapter: Baltic sea circulation. [In:] Encyclopedia of Ocean Sciences.
https://doi.org/10.1006/rwos.2001.0381
Lacy, J.R., Rubin, D.M., Ikeda, H., Mokudai, K., Hanes, D.M., 2007.
Bed
forms created by simulated waves and currents in a large flume. J.
Geophys. Res. 112, C10018.
https://doi.org/10.1029/2006JC003942
Lim, K.Y., Madsen, O.S., 2016.
An experimental study on near-orthogonal
wave–current interaction over smooth and uniform fixed roughness beds.
Coast. Eng. 116, 258–274.
https://doi.org/10.1016/j.coastaleng.2016.05.005
Malarkey, J., Davies, A.G., 1998. Modelling wave–current interactions
in rough turbulent bottom boundary layers. Ocean Eng. 25, 119–141. https://doi.org/10.1016/S0029-8018(96)00062-5
Meyer, Z., 2009. Modified Logarithmic Tachoida Applied to Sediment
Transport in a River. Acta Geophysica 57 (3), 743–759. https://doi:10.2478/s11600-009-0010-0
Nielsen, P., 1992. Coastal bottom boundary layers and sediment
transport. Advanc. Ser. Ocean Eng., Vol 4, World Sci. Publ. Co. Pte. Ltd.,
340 pp. https://doi.org/10.1142/1269
Nielsen, P., 2009. Coastal and Estuarine Processes. Advanc. Ser. Ocean
Eng., Vol 29, World Sci. Publ. Co. Pte. Ltd., 343 pp. https://doi.org/10.1142/7114
Ostrowski, R., Schönhofer, J., Szmytkiewicz, P., 2016. South Baltic
representative coastal field surveys, including monitoring at the Coastal
Research Station in Lubiatowo, Poland. J. Marine Syst. 162, 89–97. https://doi.
org/10.1016/j.jmarsys.2015.10.006
Ostrowski, R., Stella, M., 2020. Potential dynamics of nontidal sea bed in
remote foreshore under waves and currents. Elsevier Science B.V., 207 pp.
https://doi.org/10.1016/j.oceaneng.2020.107398
Ostrowski, R., Stella-Bogusz, M., 2023. Modified logarithmic distribution
of wind-driven flow velocity in remote foreshore of the non-tidal sea.
Oceanologia, 65 (4), 556–563. https://doi.org/10.1016/j.oceano.2023.06.003
Ostrowski, R., Stella, M., Szmytkiewicz, P., Kapiński, J., Marcinkowski, T.,
2018. Coastal hydrodynamics beyond the surf zone of the south Baltic
Sea. Oceanologia, 60 (3), 264–276. https://doi.org/10.1016/j.oceano.2017.11.007
Overes, P.H.P., Borsje, B.W., Luijendijk, A.P., Hulscher, S.J.M.H., 2024.
The importance of time-varying, nontidal currents in modelling in-situ sand
wave dynamics. Coast. Eng. 189, 104480. https://doi.org/10.1016/j.coastaleng.2024.104480
Pruszak, Z., Szmytkiewicz, P., Ostrowski, R., Skaja, M., Szmytkiewicz, M.,
2008. Shallow-water wave energy dissipation in a multi-bar coastal
zone. Oceanologia, 50 (1), 43–58.
Reyes-Hernández, C., Valle-Levinson, A.. 2010. Wind Modifications to
Density-Driven Flows in Semienclosed, Rotating Basins. J. Phys. Oceanogr.
40, 1473–1487. https://doi.org/10.1175/2010JPO4230.1
Stella, M., 2021. Morphodynamics of the south Baltic seabed in the remote
nearshore zone in the light of field measurements. Mar. Geol. https://doi.org/10.1016/j.margeo.2021.106546
Stella, M., Ostrowski, R., Szmytkiewicz, P., Kapiński, J., Marcinkowski, T.,
2019. Driving forces of sandy sediment transport beyond the surf zone.
Oceanologia, 61 (1), 50–59. https://doi.org/10.1016/j.oceano.2018.06.003
Trzeciak, S., 2000. Marine Meteorology with Oceanography. Wyd. Nauk.
PWN, 249 pp., (in Polish).
van Rijn, L.C., 1993. Principles of Sediment Transport in Rivers, Estuaries
and Coastal Seas. Vol. 1006. Aqua Publ., Amsterdam.
Wiberg, P.L., 2005. Wave-Current Interaction. [In:] Encyclopedia of
Coastal Science, Schwartz, M.L. (Ed.), Encyclopedia Earth Sci. Ser., Springer,
Dordrecht. https://doi.org/10.1007/1-4020-3880-1_343
Zuo, L.Q., Roelvink, D., Lu, Y.J., 2019. The mean suspended sediment
concentration profile of silty sediments under wave-dominant conditions.
Cont. Shelf Res. 186, 111–126. https://doi.org/10.1016/j.csr.2019.07.016
Diel patterns of fish activity in the Vistula Lagoon’s littoral zone: integrating acoustic and net fishing methods
Oceanologia, 67 (4)/2025, 67403, 9 pp.
https://doi.org/10.5697/FOBG2231
Beata Schmidt*, Ryszard Kornijów
Department of Fisheries, Oceanography and Marine Ecology, National Marine Fisheries Research Institute, Gdynia, Poland;
e-mail: bschmidt@mir.gdynia.pl (Beata Schmidt)
*corresponding author
Keywords:
Vistula Lagoon; Sonar ARIS; Horizontal fish migrations
Received: 28 October 2024; revised: 19 August 2025; accepted: 16 September 2025
Highlights
- Roach, bleak, silver bream, and juvenile perch were the main catches.
- Fish increased their activity during the day, with morning reverse migrations.
- ARIS sonar detected more fish than nets, highlighting method differences.
Abstract
The study investigated summer diel variability in fish abundance and movement within a highly eutrophic Baltic lagoon,
with a focus on horizontal migrations between reed beds and open water. A combination of multibeam ARIS sonar
and traditional net fishing was employed, allowing for continuous, non-invasive monitoring of fish behaviour alongside
assessments of species composition and size structure. The dominant species caught included roach, bleak, silver
bream, and juvenile perch. Acoustic recordings revealed consistently higher fish activity during the day compared to the
night, with activity elevated during the full moon relative to the new moon. Morning migrations to open water were
observed, whereas mass evening migrations back to the reed beds occurred only once. The study suggests the presence
of reverse migration patterns, potentially driven by improved visibility at night and predation pressure from sander.
References 
Appelberg, M., Berger, H.M., Hesthagen, T., Kleiven, E., Kurkilahti, M.,
Raitaniemi, J., Rask, M., 1995.
Development and intercalibration of methods
in Nordic freshwater fish monitoring. Water Air Soil Pollut. 85,
401–406.
https://doi.org/10.1007/BF00476862
Benndorf, J., 1990.
Conditions for effective biomanipulation: conclusions
derived from whole-lake experiments in Europe. Hydrobiologia 200/201,
187–203.
https://doi.org/10.1007/BF02530339
Bollens, S.M., Frost, B.W., Thoreson, D.S., Watts, S.J., 1992.
Diel
vertical migration in zooplankton: field evidence in support of the predator
avoidance hypothesis. Hydrobiologia 234, 33–39.
Brabrand, Å., Faafeng, B., 1993.
Habitat shift in roach (Rutilus rutilus)
induced by pikeperch (Stizostedion lucioperca) introduction: predation risk
versus pelagic behaviour. Oecologia 95, 38–46.
https://doi.org/10.1007/BF00649504
Cervetto, G., Pagano, M., Gaudy, R., 1995.
Feeding behaviour and migrations
in a natural population of the copepod Acartia tonsa. Hydrobiologia 300,
237–248.
https://doi.org/10.1007/BF00024464
Gaudreau, N., Boisclair, D., 2000.
Influence of moon phase on acoustic
estimates of the abundance of fish performing daily horizontal migration in a
small oligotrophic lake. Can. J. Fish. Aquat. Sci. 57, 581–590.
https://doi.org/10.1139/f99-277
Gliwicz, Z.M., Jachner, A., 1992.
Diel migrations of juvenile fish – a
ghost of predation past or present. Arch. Hydrobiol. 124, 385–410.
https://doi.org/10.1127/archiv-hydrobiol/124/199
2/385
Gliwicz, Z.M., Slon, J., Szynkarczyk, I., 2006. Trading safety for
food: evidence from gut contents in roach and bleak captured at different
distances offshore from their daytime littoral refuge. Fresh. Biol. 51,
823–839. https://doi.org/10.1111/j.1365-2427.2006.01530.x
Horppila, J., Liljendahl, A., Estlander, S., Nurminen, L., 2018. The role
of visual and physiological refuges in humic lakes: Effects of oxygen, light
quantity, and spectral composition on daytime depth of chaoborids. Int.
Rev. Hydrobiol. 103, 63–70. https://doi.org/10.1002/iroh.201801933
Hӧlker, F., Haertel, S. S., Steiner, S., Mehner, T., 2002. Effects of
piscivore-mediated habitat use on growth, diet and zooplankton consumption of
roach: an individual-based modelling approach. Freshw. Biol. 47,
2345–2358. https://doi.org/10.1046/j.1365-2427.2002.01002.x
Imbrock, F., Appenzeller, A., Eckmann, R., 1996. Diel and seasonal
distribution of perch in Lake Constance: A hydroacoustic study and in situ
observations. J. Fish Biol. 49, 1–13. https://doi.org/10.1111/j.1095-8649.1996.tb00001.x
Jacobsen, L., Berg, S., Jepsen, N., Skov, C., 2004. Does roach behaviour
differ between shallow lakes of different environmental state? J. Fish
Biol. 65, 135–147. https://doi.org/10.1111/j.0022-1112.2004.00436.x
Järvalt, A., Krause, T., Palm, A., 2005. Diel migration and spatial
distribution of fish in a small stratified lake. Hydrobiologi 547,
197–203. https://doi.org/10.1007/s10750-005-4160-z
Jeppesen, E., Pekcan-Hekim, Z., Lauridsen, T.L., Søndergaard, M., Jensen,
J.P., 2006. Habitat distribution of fish in late summer: changes along a
nutrient gradient in Danish lakes. Ecol. Freshw. Fish 15, 180–190. https://doi.org/10.1111/j.1600-0633.2006.00142.x
Karpowicz, M., Kornijów, R., Ejsmont-Karabin, J., 2023. Not a good place
to live for most, excellent for a few - diversity of zooplankton in a shallow
coastal ecosystem. Sustainability 15(3), 2345. https://doi.org/10.3390/su15032345
Kornijów, R., 2018. Ecosystem of the Polish part of the Vistula Lagoon
from the perspective of alternative stable states concept, with implications
for management issues. Oceanologia 60(3), 390–404. https://doi.org/10.1016/j.oceano.2018.02.004
Kornijów, R., Karpowicz, M., Ejsmont-Karabin, J., Nawrocka, L., de Eyto, E.,
Grzonkowski, K., Magnuszewski, A., Jakubowska, A., Wodzinowski, T.,
Woźniczka, A., 2020. Patchy distribution of phyto- and zooplankton in
large and shallow lagoon under ice cover and resulting trophic
interactions. Mar. Freshwater Res. 71, 1327–1341. https://doi.org/10.1071/MF19259
Martignac, F., Daroux, A., Bagliniere, J.L., Ombredane, D., Guillard, J., 2015.
The use of acoustic cameras in shallow waters: New hydroacoustic tools for
monitoring migratory fish population. A review of DIDSON
technology. Fish Fish. 16, 486–510. https://doi.org/10.1111/faf.12071
Moss, B., 1994. Brackish and freshwater shallow lakes – different systems or
variations on the same theme? Hydrobiologia 275, 1–14. https://doi.org/10.1007/BF00026695
Moursund R.A., Carlson T.J., Peters R.D., 2003. A fisheries application of
dual-frequency identification sonar acoustic camera, ICES J. Mar. Sci. 60,
678–683. https://doi.org/10.1016/S1054-3139(03)00036-5
Muška, M., Tušer, M., Frouzová, J. Drastı́k, V., Cech, M., Juza, T.,
Kratochvı́l, M., Mrkvicka, T., Peterka, J., Prchalová, M., Rı́ha, M.,
Vasek, M., Kubecka, J., 2013. To migrate, or not to migrate: partial diel
horizontal migration of fish in a temperate freshwater reservoir.
Hydrobiologia 707, 17–28. https://doi.org/10.1007/s10750-012-1401-9
Olin, M., Kurkilahti, M., Peitola, P., Ruuhijärvi, J., 2004. The effects
of fish accumulation on the catchability of multimesh gillnet. Fish. Res.
68: 135–147. https://doi.org/10.1016/j.fishres.2004.01.005
Pawlikowski, K., Kornijów, R., 2019. Role of macrophytes in structuring
littoral habitats in the Vistula Lagoon (southern Baltic Sea). Oceanologia
61(1), 26–37. https://doi.org/10.1016/j.oceano.2018.05.003
Pekcan-Hekim, Z., Nurminen, L., Ojala, T., Olin, M., Ruuhijärvi, J.,
Horppila, J., 2010. Reversed Diel HorizontalMigration of Fish: Turbidity
Versus Plant Structural Complexity as Refuge. J. Freshw. Ecol., 25(4),
649–656. https://doi.org/10.1080/02705060.2010.9664414
Psuty-Lipska, I., Borowski, W., 2003. Factors affecting fish assemblages in
the Vistula Lagoon. Arch. Fish. Mar. Res. 50(3), 253–270.
Psuty, I., Wilkońska, H., 2009. The stability of fish assemblages under
unstable conditions: A ten-year series from the Polish part of the Vistula
Lagoon. Arch. Pol. Fisheries 17. https://doi.org/10.2478/v10086-009-0004-1
Ravn, H.D., Lauridsen, T.L., Jepsen, N., Jeppesen, E., Hansen, P.G., Hansen,
J.G., Berg, S., 2019. A comparative study of three different methods for
assessing fish communities in a small eutrophic lake. Ecol. Freshw. Fish
28, 341–352. https://doi.org/10.1111/eff.12457
Vasek, M., Kubecka, J., 2004. In situ diel patterns of zooplankton
consumption by subadult/adult roach Rutilus rutilus, bream Abramis brama, and
bleak Alburnus alburnus. Folia Zool. 53, 203–214.
Wasserman, R.J., Vink, T.J.F., Kramer, R., Froneman, P.W., 2014.
Hyperbenthic and pelagic predators regulate alternate key planktonic
copepods in shallow temperate estuaries. Mar. Freshw. Res. 65, 791–801.
https://doi.org/10.1071/MF13233
Wolter C., Freyhof J., 2004. Diel distribution patterns of fishes in a
temperate large lowland river. J. Fish Biol. 64, 632–642. https://doi.org/10.1111/j.1095-8649.2004.00327.x
Dynamics of ice phenomena on the lake shores based on ice scars method – study from the Southern Baltic Sea coast
Oceanologia, 67 (4)/2025, 67404, 16 pp.
https://doi.org/10.5697/ZVAO7632
Józef P. Girjatowicz, Tomasz A. Łabuz*, Małgorzata Świątek
Institute of Marine and Environmental Science, University of Szczecin, Mickiewicza 16, 70–383 Szczecin, Poland;
e-mail: tomasz.labuz@usz.edu.pl (Tomasz A. Łabuz)
*corresponding author
Keywords:
Piled ice; Ice scars; Coastal lakes; Shore erosion; Baltic Sea’s coast
Received: 22 October 2024; revised: 2 September 2025; accepted: 3 September 2025
Highlights
- Extent of ice pileups determined on basis of scars on trees.
- Ice damage value increased to E by ice shifted by SW wind during weather warming.
- The greatest and farthest damage on NE coasts.
- Highest ice pileups reached 3.8 m above water level and up to 38 m from the shore.
Abstract
This paper analyses how ice pile-ups affect lake shores, how far ice thrusts reach and the height of ice pilings, while also determining the regions that are most vulnerable to the destructive impact of ice on reservoirs located in a coastal zone. This was achieved by a field survey examining ice scars on trees growing along the lakes of the southern Baltic Sea. The ice scars were mainly measured in terms of elevation and length, the distance of the damaged trees from the shore, geographic coordinates, the geographic direction the scars were facing and the elevation of the ground above
the water level. A t-test was used to determine whether the tree scars on the individual lakes varied from the average
in elevation or distance from the shore.
The furthest traces of ice pile-ups observed on trees were 38 m inland, while the maximum height of the ice piles was
3.8 m. These maximum scores were found at lakes located further east, on their northeastern shores, probably because
the most frequent and strongest winds tend to be westerly. Based on the research findings, some conclusions can be
drawn about the dynamics of ice phenomena, especially in areas where systematic observations of this type were not
carried out. The results can be used to design shore protection and may be relevant for investment plans for municipal
housing, transport and recreational infrastructure
References 
Alestalo, J., Häikiö, J., 1976
. Forms created by the thermal movement
of lake ice in Finland in winter 1972–1973. Fenia 157 (2), 51–92.
Banzhaf, W., 1931.
Ice pileups on the Szczecin Lagoon. Nature und
Museum 61 (12), 491–494 (in German).
Baranowski, D., Chlost, I., 2009.
Hydro-meteorological conditions in the
catchment of the Łebsko Lake. Baltic Coastal Zone 13, 109–119.
Barnes, P.W., Kempema., E.W., Reimnitz, E., McCormick M., 1994.
The
influence of ice on southern Lake Michigan coastal erosion. J. Great Lakes
Res. 20 (1), 179–195.
https://doi.org/10.1016/S0380-1330(94)71139-4
Bégin, Y., Payette, S., 1991.
Population structure of lakeshores willows
and ice-push events in subarctic Québec, Canada. Holarctic Ecol. 14,
9–17.
Beltaos, S., Prowse, T.D., Carter, T., 2006.
Ice regime of the lower Peace
River and ice-jam flooding of the Peace-Athabasca Delta. Hydrol. Process.
20 (19), 4009–4029.
https://doi.org/10.1002/hyp.6417
Correns, M., 1973.
Eisverhältnisse des Peenestrom – Haffgebietes – ein
Beitrag zur Hydrogrphie der Gewässer an der Sűdlichen Ostseekűstr.
Petermanus Geographischen Mitteilungen 174, 4 (in German).
Cieśliński, R., 2011
. Geographical conditions of hydrochemical
variability of lakes on the southern Baltic coast. Gdańsk Univ. Publ.,
Gdańsk (in Polish).
Cieśliński, R., Drwal, J., Chlost, I., 2009
. Sea water intrusions to
the lake Gardno. Baltic Coastal Zone 13, 85–95.
Choiński, A., 1995.
Outline of physical limnology of Poland. Wyd.
Nauk. Uniw. Adama Mickiewicza (in Polish).
Choiński, A., Ptak, M., Skowron, R., Strzelczak, A., 2015.
Changes in ice
phenology on Polish lakes from 1961 to 2010 related to location and
morphometry. Limnologica 53, 42–49.
https://doi.org/10.1016/j.limno.2015.05.005
Christensen, F.T., 1994.
Ice ridge-up and pile-up on shores and coastal
structures. J. Coast Res. 10 (3), 681–701.
Cyberski, J., Grześ, M., Gutry-Korycka, M., Nachlik, E., Kundzewicz, Z.W.,
2006.
History of floods on the River Vistula. Hydrolog. Sci. J. 51
(5), 799–817.
https://doi.org/10.1623/hysj.51.5.799
Dione, J.C., 1979.
Ice action in the lacustrine environment – A review
with particular reference to subarctic Quebec, Canada. Earth Sci. Rev. 15
(3), 185–212.
Duguay, C.R., Prowse, T.D., Bonsal, B.R., Lacroix, M.P., Mé- nard, P., 2006.
Recent trends in Canadian lakes ice cover. Hydrolog. Process. 20,
781–801.
https://doi.org/10.1002/hyp.6131
Engström, J., Jansson, R., Nilsson, C., Weber, C., 2011.
Effects of river
ice on riparian vegetation. Freshwater Biol. 56, 1095–1195.
https://doi.org/10.1111/j.1365-2427.2010.02553.
Gatto, L.W., 1984.
Reservoir Bank erosion caused by ice. Cold Reg.
Sci. Techn. 9, 203–214.
Girjatowicz, J.P., 2011. Effects of the North Atlantic
Oscillation on water
temperature in southern Baltic coastal lakes. Annales Limnol. – Int. J.
Lim. 47, 73–84.
https://doi.org/1051/Limn/2010031
Girjatowicz, J.P., 2014.
Ice thrusting and hummocking on the shores of the
Southern Baltic Sea’s coastal lagoons. J. Coast. Res. 30 (3), 456–464.
https://doi.org/10.2112/JCOASTRES-D-12-00032.1
Girjatowicz, J.P., 2015.
Forms of onshore ice thrusting in coastal lagoons
of the Southern Baltic Sea. J. Cold Reg. Eng. 29 (1), 1–17.
https://doi.org/10.1061/(ASCE)CR.1943-5495.0000069
Girjatowicz, J.P., Łabuz, T. A., 2020.
Forms of piled ice at the southern
coast of the Baltic Sea. Est. Coast. Shelf Sci. 239.
https://doi.org/10.1016/j.ecss.2020.106746
Girjatowicz, J.P., Świątek, M., Kowalewska-Kalkowska, H., 2022.
Relationships between air temperature and conditions on the southern Baltic
coastal lakes in the context of climate change. J. Limn. 81, 2060.
https://doi.org/10.4081/jlimnol.2022.2060
Girjatowicz, J.P., Świątek, M., Łabuz, T.A., 2024.
The range of sliding
and piling ice on the basis of ice scars on trees in coast of the coastal
lagoons on the example of the Szczecin Lagoon. Quaest. Geogr. 43 (1),
93–110.
https://doi.org/10.14746/quageo-2024-0006
Haapala, J., Ronkainen, I., Schmelzer, N., Sztobryn, M., 2015.
Recent
change – sea ice. [In:] The BACC II Author Team (Eds.), Second assessment of
climate change for the Baltic Sea basin, International Baltic Sea
Secretariat, Springer, Geesthacht, 145–153.
Jańczak, J., 1997.
Atlas of Polish Lakes. Wyd. Inst. Meteorol. Gosp.
Wod., Poznań (in Polish).
Jevrejeva, S., Drabkin, V.V., Kostjukov, J., Lebedev, A.A., Lepparänta, M.,
Mironov, Y.U., Schmelzer, N., Sztobryn, M., 2004.
Baltic Sea season in the
twentieth century. Climate Res. 25 (23), 217–227.
https://doi.org/10.3354/cr025217
Kaptein, M., van den Heuvel, E., 2022.
Statistics for data scientists: an
introduction to probability, statistics and data analysis. Springer,
Berlin, Heidelberg, New York.
Kozlov, I.E., Krek, E.V., Kostianoy, A.G., Dailidienė, I., 2020.
Remote
sensing of ice conditions in the southeastern Baltic Sea and in the Curonian
Lagoon and validation of SAR-based ice thickness products. Remote Sens.
12(20), 3754, 20 pp.
https://doi.org/10.3390/rs12223754
Kraus, E., 1930.
Over ice pileups. III Hydrological Conference of the
Baltic Countries, Warsaw, Poland, 1–44 (in German).
Leckebusch, G.C., Ulbrich, W., 2004.
On the relationship between cyclones
and extreme windstorm events over Europe under climate change. Global
Planet. Change 44, 181–193.
https://doi.org/10.1016/j.gloplacha.2004.06.011
Lederer, J.R., Garver, J.I., 1996.
Ice jams inferred from tree scars made
during the 1996 mid-winter flood on the Mohawk River. Geology Department
Union College, New York, USA.
Lederer, J.R., Garver, J.I., 2000.
Ice jams on the lower Mohawk River
(Crescent, NY) formed during the 2000 mindwinter flood. Geology Department
Union College, New York, USA.
Lemay, M., Bégin, Y., 2012.
Using ice-scars as indicators of exposure to
physical lakeshore disturbances, Corvette Lake, northern Quebec, Canada.
Earth Surface Processes and Landforms 37 (13), 1353–1361.
https://doi.org/10.1002/esp.3244
Leppäranta, M., 2010.
Modelling of formation and decay of lake ice. [In:]
George, G., (Ed.), The impact of climate change on European lakes,
Springer, 63–83.
https://doi.org/10.1007/978-90-481-2945-4_5
Leppäranta, M., 2013.
Land-ice interaction in the Baltic Sea.
Estonian J. Earth Sci. 62 (1), 2–14.
https://doi.org/10.3176/earth.2013.01
Leppäranta, M., Myrberg, K., 2009.
Physical Oceanography of the Baltic
Sea. Springer.
https://doi.org/10.1007/978-3-540-79703-6
Lind, L.C., Nilsson, C., Povli, L.E., Weber, C., 2014.
The role of ice
dynamics in shaping vegetation in flowing waters. Biolog. Rev. 89,
791–804.
https://doi.org/10.1111/brv.12077
Livingstone, D.M., Adrian, R., Blenckner, T., George. G., Weyhenmeyer, G.A.,
2009.
Lake ice phenology. [In:] George, G. (Ed.), The impact of climate
change on European lakes. Aquat. Ecol. Ser. Vol. 4, Springer, Dordrecht,
51–61.
https://doi.org/10.1007/978-90-481-2945-4_4
Łabuz, T.A., 2019.
Storm surges in the 21st century (2000–2019) and
their impact on the erosion of the dune coast in Poland, 28–29. [In:]
Abstracts of 2nd Scientific Conference of Polish Sea Researchers: State and
trends in seas and oceans, Gdynia (Poland) 24-25.09.2019.
Orviku, K., Jaagus, J., Tõnisson, H., 2011.
Sea ice shaping the
shores. J. Coast. Res. 64 (Sp. Iss.), 681–685.
https://www.jstor.org/stable/26482258
Pawłowski, B., 2019.
Ice jams: causes and effects. [In:] Maurice, P.A.
(Ed.), Encyclopedia of water: science, technology and society. John Willey
& Sons Inc.
https://doi.org/10.1002/9781119300762
Pyökäri, M., 2011
. Ice action on lake shores near Schefferville,
central Quebec – Labrador, Canada. Can. J Earth Sci. 18 (10),
1629–1634.
https://doi.org/10.1139/e81-149
Reinhard, H., 1955.
Eispressungen an der Kũste. Weissenschaftliche
Zeitschrift der Ernst Moritz Arndt Universität Greifswald, Jahr. 4, 6/7
(in German). Saart, P., 2022. Peipus Lake.
https://www.youtube.com/watch?v=SYIrxoY08zs
Schönwiese, Ch., Rapp, J., 1997.
Climate trend atlas of Europe based on
observations 1891–1990. Kluwer Acad. Publ.
Sepp, M., Post, P., Jaagus, J., 2005.
Long-term changes in the frequency of
cyclones and their trajectories in Central and Northern Europe. Nordic
Hydrol. 36, 297–309.
https://doi.org/10.2166/nh.2005.0023
Skowron, R., 2009.
Changeability of the ice cover on the lakes of northern
Poland in the light of climatic changes. Bull. Geogr. Phys., Geogr. Ser. 1
(1), 103–124.
https://doi.org/10.2478/bgeo-2009-0007
Smith, D.G., Reynolds, D.M., 1983.
Trees scars to determine the frequency
and stage of high magnitude river ice drives and jams, Red Deer, Alberta.
Can. Water Res. J. 8 (3), 77–94.
Sui, J., Karney, B.W., Fang, D., 2005. Variation in water level under
ice-jammed conditions – field investigation and experimental study.
Nordic Hydrol. 36, 65–84.
Sztobryn, M., Wójcik, R., Miętus, M., 2012. Ice cover in the Baltic Sea
– current status and expected changes in the future. [In:] Climatic and
oceanographic conditions in Poland and the Southern Baltic Sea. Expected
changes and guidelines for the development of adaptation strategies in the
national economy, Monograph Ser., IMGW-PIB Publ., 189–215.
Tarnowska, K., 2011. Strong winds on the Polish coast of the Baltic
Sea, Prac. Stud. Geogr. WGiSR UW 47, 197–204 (in Polish).
Tomczyk, A., Bednorz, E., (Ed.), 2022. Climate atlas of Poland
(1991–2020). Bogucki Sci. Publ. (in Polish).
Uunila, R., Church, M., 2014. Ice on Peace River: Effects on Bank
Morphology and Riparian Vegetation. [In:] Church, M. (Ed.), The regulation of
Peace River: a case study for river management. Willey & Sons Inc.,
391–420. https://doi.org/10.1002/9781118906170.ch6
Vandermause, R., Harvey, M., Zevenbergen, L., Ettema, R., 2021. River-ice
effects on bank erosion along the middle segment of the Susitna River,
Alaska. Cold. Reg. Sci. Techn. 185, 103239. https://doi.org/10.1016/j.coldregions.2021.103239
Wibig, J., 2021. Change of wind. [In:] Falarz, M., (Ed.), Climate change in
Poland. Past, present, future. Springer, 391–420. https://doi.org/10.1007/978-3-030-70328-8
Wiśniewski, B., Wolski, T., 2011. Physical aspects of extreme storm
surges and falls on the Polish coast. Oceanologia 53 (1 TI), 373–390. https://doi.org/10.5697/oc.53-1-TI.373.
Wrzesiński, D., Choiński, A., Ptak, M., Skowron, R., 2015. Effect of
the North Atlantic Oscillation on the pattern of lake ice Phenology in
Poland. Acta Geophys. 63, 1664–1684. https://doi.org/10.1515/acgeo-2015-0055
Decadal change of benthic macroinvertebrates driven by multiple stresses in the Changjiang Estuary in summer
Oceanologia, 67 (4)/2025, 67405, 15 pp.
https://doi.org/10.5697/KFHM6788
Yanbin Tang1,2,4, Bin Wang1,2, Dewang Li1,2, Xiao Ma2,3, Zhibing Jiang1,2, Yibo Liao1,2, Qinghe Liu1,2, Rongliang Zhang1,2, Jiangning Zeng1,2, Jianfang Chen1,2,3, Chenghua Li4,*, Lu Shou1,2,*
1Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, P. R. China;
e-mail: shoulu981@sio.org.cn (L. Shou), lichenghua@nbu.edu.cn (Ch. Li)
2Observation and Research Station of Yangtze River Delta Marine Ecosystems, Ministry of Natural Resources, Zhoushan 316022, P. R. China
3State Key Laboratory of Satellite Ocean Environment Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, P. R. China
4School of Marine Sciences, Ningbo University, Ningbo 315211, P. R. China
*corresponding author
Keywords:
Benthic macroinvertebrate; Large estuary; Diluted water; Anthropogenic activity; El Niño
Received: 26 November 2024; revised: 16 September 2025; accepted: 23 September 2025
Highlights
- Both human activities and climate change influence benthic macroinvertebrates in the Changjiang Estuary.
- The abundance and biomass of benthic macroinvertebrates increased significantly from 2006 to 2014 due to a series of environmental protection measures.
- Regional protective measures were overwhelmed by intensive El Niño events.
- International cooperation is required to protect estuarine ecosystems under scenarios of global climate change.
Abstract
Both human activities and climate change have influenced benthic macroinvertebrates in the Changjiang Estuary since the Anthropocene. As a result, we investigated long-term variations in benthic macroinvertebrates and related them to changes in depth, salinity, temperature, pH and dissolved oxygen in bottom water off the Changjiang Estuary from 10 summer cruises during 2006–2021. The bi-monthly multivariate ENSO index and summer runoff rate of Changjiang were used to estimate the climate change during this period. The abundance and biomass of benthic macroinvertebrates increased significantly from 2006 to 2014 owing to a series of environmental protection measures. An intensive El Niño event, coupled with Pacific Decadal Oscillation (PDO) and Atlantic Multidecadal Oscillation (AMO), promoted diluted water discharge and hypoxia in summer in the Changjiang Estuary since 2015. We noted changes in the macrobenthic community following these events, including a dramatic decrease in abundance and biomass, alterations in dominant species and a decline in benthic diversity. Correlation analysis, canonical correspondence and redundancy analysis revealed that depth, salinity and dissolved oxygen were the main factors influencing the distribution of benthic macroinvertebrates. Owing to the ubiquitous pressure caused by human activities and climate change in estuaries, we make an appeal that international cooperation is required to protect estuarine ecosystems under the scenario of global climate change.
References 
Abele, D., Großpietsch, H., Pörtner, H.O., 1998.
Temporal fluctuations
and spatial gradients of environmental P(O2), temperature, H2O2 and H2S in its
intertidal habitat trigger enzymatic antioxidant protection in the capitellid
worm Heteromastus filiformis. Mar. Ecol. Prog. Ser. 163, 179–191.
https://doi.org/10.3354/meps163179
Adeleye, A.O., Jin, H., Di, Y., Li, D., Chen, J., Ye, Y., 2016.
Distribution and ecological risk of organic pollutants in the sediments and
seafood of Yangtze Estuary and Hangzhou Bay, East China Sea. Sci. Total
Environ. 541, 1540–1548.
https://doi.org/10.1016/j.scitotenv.2015.09.124
Aller, J.Y., Aller, R.C., 1986.
General characteristics of benthic faunas
on the Amazon inner continental shelf with comparison to the shelf off the
Changjiang River, East China Sea. Cont. Shelf Res. 6, 291–310.
https://doi.org/10.1016/0278-4343(86)90065-8
Arntz, W.E., Gallardo, V.A., Gutiérrez, D., Isla, E., Levin, L.A., Mendo, J.,
Neira, C., Rowe, G.T., Tarazona, J., Wolff, M., 2006.
El Niño and similar
perturbation effects on the benthos of the Humboldt, California, and Benguela
Current upwelling ecosystems. Adv. Geosci. 6, 243–265.
https://doi.org/10.5194/adgeo-6-243-2006
Bett, P.E., Scaife, A.A., Li, C., Hewitt, C., Golding, N., Zhang, P., Dunstone,
N., Smith, D.M., Thornton, H.E., Lu, R., Ren, H.L., 2018.
Seasonal
forecasts of the summer 2016 Yangtze River basin rainfall. Adv. Atmos.
Sci. 35, 918–926.
https://doi.org/10.1007/s00376-018-7210-y
Bouloubassi, I., Fillaux, J., Saliot, A., 2001.
Hydrocarbons in surface
sediments from there Changjiang (Yangtze River) Estuary, East China Sea.
Mar. Pollut. Bull. 42, 1335–1346.
https://doi.org/10.1016/S0025-326X(01)00149-7
Chao, M., Shi, Y., Quan, W., Shen, X., An, C., Yuan, Q., Huang, H., 2012.
Distribution of benthic macroinvertebrates in relation to environmental
variables across the Yangtze River Estuary, China. J. Coast. Res. 28,
1008–1019.
https://doi.org/10.2112/JCOASTRES-D-11-00194.1
Chen, T., Wang, Y., Gardner, C., Wu, F., 2020.
Threats and protection
policies of the aquatic biodiversity in the Yangtze River. J. Nat.
Conserv. 58, 125931.
https://doi.org/10.1016/j.jnc.2020.125931
Cheung, R.C.W., Yasuhara, M., Iwatani, H., Wei, C.L., Dong, Y.W., 2019.
Benthic community history in the Changjiang (Yangtze River) mega-delta:
Damming, urbanization, and environmental control. Paleobiology 45,
469–483.
https://doi.org/10.1017/pab.2019.21
Currie, D.R., Small, K.J., 2005.
Macrobenthic community responses to
long-term environmental change in an east Australian sub-tropical estuary.
Estuar. Coast. Shelf Sci. 63, 315–331.
https://doi.org/10.1016/j.ecss.2004.11.023
Dafforn, K.A., Kelaher, B.P., Simpson, S.L., Coleman, M.A., Hutchings, P.A.,
Clark, G.F., Knott, N.A., Doblin, M.A., Johnston, E.L., 2013.
Polychaete
richness and abundance enhanced in anthropogenically modified estuaries despite
high concentrations of toxic contaminants. PLoS One 8, e77018.
https://doi.org/10.1371/journal.pone.0077018
Dai, S.B., Lu, X.X., 2014.
Sediment load change in the Yangtze River
(Changjiang): A review. Geomorphology 215, 60–73.
https://doi.org/10.1016/j.geomorph.2013.05.027
Dauvin, J.C., 2007.
Paradox of estuarine quality: Benthic indicators and
indices, consensus or debate for the future. Mar. Pollut. Bull. 55,
271–281.
https://doi.org/10.1016/j.marpolbul.2006.08.017
de-la-Ossa-Carretero, J.A., Del-Pilar-Ruso, Y., Loya-Fernández, A.,
Ferrero-Vicente, L.M., Marco-Méndez, C., Martinez-Garcia, E.,
Giménez-Casalduero, F., Sánchez-Lizaso, J.L., 2016
. Bioindicators as
metrics for environmental monitoring of desalination plant discharges.
Mar. Pollut. Bull. 103, 313–318.
https://doi.org/10.1016/j.marpolbul.2015.12.023
de Lima, T.M., Nery, L.E.M., Maciel, F.E., Ngo-Vu, H., Kozma, M.T., Derby,
C.D., 2021.
Oxygen sensing in crustaceans: functions and mechanisms.
J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol. 207, 1–15.
https://doi.org/10.1007/s00359-020-01457-z
Diaz, R.J., Rosenberg, R., 1995.
Marine benthic hypoxia: a review of its
ecological effects and the behavioural responses of benthic macrofauna.
Oceanogr. Mar. Biol. Annu. Rev. 33, 245–303.
Dolbeth, M., Cardoso, P.G., Grilo, T.F., Bordalo, M.D., Raffaelli, D., Pardal,
M.A., 2011.
Long-term changes in the production by estuarine macrobenthos
affected by multiple stressors. Estuar. Coast. Shelf Sci. 92, 10–18.
https://doi.org/10.1016/j.ecss.2010.12.006
Dong, A., Zhai, S., Matthias, Z., Yu, Z., Zhang, H., Liu, F., 2012.
Heavy
metals in Changjiang estuarine and offshore sediments: Responding to human
activities. Acta Oceanol. Sin. 31, 88–101.
https://doi.org/10.1007/s13131-012-0195-y
Douglas, E.J., Bulmer, R.H., MacDonald, I.T., Lohrer, A.M., 2022.
Estuaries
as coastal reactors: importance of shallow seafloor habitats for primary
productivity and nutrient transformation, and impacts of sea level rise.
New Zeal. J. Mar. Freshw. Res. 56 (3), 553–569.
https://doi.org/10.1080/00288330.2022.2107027
Duan, X., Liu, J., Zhang, D., Yin, P., Li, Y., Li, X., 2015.
An assessment
of human influences on sources of polycyclic aromatic hydrocarbons in the
estuarine and coastal sediments of China. Mar. Pollut. Bull. 97,
309–318.
https://doi.org/10.1016/j.marpolbul.2015.05.071
Elliott, M., Quintino, V., 2007.
The Estuarine Quality Paradox,
Environmental Homeostasis and the difficulty of detecting anthropogenic stress
in naturally stressed areas. Mar. Pollut. Bull. 54, 640–645.
https://doi.org/10.1016/j.marpolbul.2007.02.003
Fang, C., Liu, Y., Cai, Q., Song, H., 2021.
Why does extreme rainfall occur
in central China during the summer of 2020 after a weak El Niño? Adv.
Atmos. Sci. 8, 2067–2081.
https://doi.org/10.1007/s00376-021-1009-y
Gammal, J., Norkko, J., Pilditch, C.A., Norkko, A., 2017.
Coastal hypoxia
and the importance of benthic macrofauna communities for ecosystem
functioning. Estuar. Coast. 40, 457–468.
https://doi.org/10.1007/s12237-016-0152-7
Ge, Y., Miao, J., Lang, X., Si, D., Jiang, D., 2023.
Combined impacts of
the Pacific Decadal Oscillation and Atlantic Multidecadal Oscillation on summer
precipitation in Eastern China during the Medieval Climate Anomaly and Little
Ice Age. J. Geophys. Res. Atmos. 128.
https://doi.org/10.1029/2023JD038920
Ghribi, R., Correia, A.T., Elleuch, B., Nunes, B., 2019.
Toxicity
assessment of impacted sediments from southeast coast of Tunisia using a
biomarker approach with the polychaete Hediste diversicolor. Arch.
Environ. Contam. Toxicol. 76, 678–691.
https://doi.org/10.1007/s00244-019-00611-2
Grothe, P.R., Cobb, K.M., Liguori, G., Di Lorenzo, E., Capotondi, A., Lu, Y.,
Cheng, H., Edwards, R.L., Southon, J.R., Santos, G.M., Deocampo, D.M.,
Lynch-Stieglitz, J., Chen, T., Sayani, H.R., Thompson, D.M., Conroy, J.L.,
Moore, A.L., Townsend, K., Hagos, M., O’Connor, G., Toth, L.T., 2020.
Enhanced El Niño–Southern Oscillation variability in recent
decades. Geophys. Res. Lett. 47, 1–8.
https://doi.org/10.1029/2019GL083906
Herman, P.M.J., Middelburg, J.J., van de Koppel, J., Heip, C.H.R., 1999.
Ecology of estuarine macrobenthos. Adv. Ecol. Res. 29, 195–240.
https://doi.org/10.1016/S0065-2504(08)60194-4
Jiang, Z., Liu, J., Chen, J., Chen, Q., Yan, X., Xuan, J., Zeng, J., 2014.
Responses of summer phytoplankton community to drastic environmental
changes in the Changjiang (Yangtze River) estuary during the past 50
years. Water Res. 54, 1–11.
https://doi.org/10.1016/j.watres.2014.01.032
Li, F., Ma, Y., Song, X., Li, S., Zhang, X., Wang, X., Wang, T., Sun, Z., 2022.
Community structure and ecological quality assessment of macrobenthos in
the coastal sea areas of Northern Yantai, China. Front. Mar. Sci. 9,
1–14.
https://doi.org/10.3389/fmars.2022.989034
Li, H.M., Tang, H.J., Shi, X.Y., Zhang, C.S., Wang, X.L., 2014.
Increased
nutrient loads from the Changjiang (Yangtze) River have led to increased
Harmful Algal Blooms. Harmful Algae 39, 92–101.
https://doi.org/10.1016/j.hal.2014.07.002
Liao, Y., Shou, L., Jiang, Z., Tang, Y., Du, P., Zeng, J., Chen, Q., Yan, X.,
Chen, J., 2019.
Effects of fish cage culture and suspended oyster culture
on macrobenthic communities in Xiangshan Bay, a semi-enclosed subtropical bay
in eastern China. Mar. Pollut. Bull. 142, 475–483.
https://doi.org/10.1016/j.marpolbul.2019.03.065
Liao, Y., Shou, L., Tang, Y., Zeng, J., Gao, A., Chen, Q., Yan, X., 2017.
Macrobenthic assemblages of the Changjiang River estuary (Yangtze River,
China) and adjacent continental shelf relative to mild summer hypoxia.
Chinese J. Oceanol. Limnol. 35, 481–488.
https://doi.org/10.1007/s00343-017-5285-4
Llansó, R.J., 1991.
Tolerance of low dissolved oxygen and hydrogen
sulfide by the polychaete Streblospio benedicti (Webster). J. Exp. Mar.
Bio. Ecol. 153, 165–178.
https://doi.org/10.1016/0022-0981(91)90223-J
Luo, B., Shen, H., 1994.
Three Gorges Project and Estuarine Ecological
Environment. Science Press, Beijing. Lv, W., Zhou, W., Zhao, Y., 2018.
Macrobenthos functional groups as indicators of ecological restoration in
reclaimed intertidal wetlands of China’s Yangtze Estuary. Reg. Stud. Mar.
Sci. 22, 93–100.
https://doi.org/10.1016/j.rsma.2018.06.003
Ma, X., Liu, A., Zhao, Q., Wang, B., Tian, D., Meng, Q., Zeng, D., Li, J.,
Huang, D., Zhou, F., 2022.
Temporal variation of summer hypoxia off
Changjiang Estuary during 1997–2014 and its association with ENSO.
Front. Mar. Sci. 9, 1–14.
https://doi.org/10.3389/fmars.2022.897063
Meng, W., Liu, L., Zheng, B., Li, X., Li, Z., 2007.
Macrobenthic community
structure in the Changjiang Estuary and its adjacent waters in summer.
Acta Oceanol. Sin. 26, 62–71.
Milliman, J.D., Huang-ting, S., Zuo-sheng, Y., H. Mead, R., 1985.
Transport
and deposition of river sediment in the Changjiang estuary and adjacent
continental shelf. Cont. Shelf Res. 4, 37–45.
https://doi.org/10.1016/0278-4343(85)90020-2
Ni, G., Kern, E., Dong, Y.W., Li, Q., Park, J.K., 2017.
More than meets the
eye: The barrier effect of the Yangtze River outflow. Mol. Ecol. 26,
4591–4602.
https://doi.org/10.1111/mec.14235
Pak, S. Il, Oh, T.H., 2010.
Correlation and simple linear regression.
J. Vet. Clin. 27, 427–434.
https://doi.org/10.4324/9781003442011-24
Peng, S., Li, X., Wang, H., Zhang, B., 2014.
Macrobenthic community
structure and species composition in the Yellow Sea and East China Sea in
jellyfish bloom. Chinese J. Oceanol. Limnol. 32, 576–594.
https://doi.org/10.1007/s00343-014-3068-8
Pinto, I., Rodrigues, S., Antunes, S.C., 2021.
Assessment of the benthic
macroinvertebrate communities in the evaluation of the water quality of
portuguese reservoirs: An experimental approach. Water (Switzerland) 13.
https://doi.org/10.3390/w13233391
Qi, W., Wang, X., Kang, J., Bai, Y., Bian, R., Xue, H., Chen, L., Guan, A.,
Pan, Y.-R., Liu, H., Qu, J., 2022.
Improvement of the Yangtze River’s
water quality with substantial implementation of wastewater services
infrastructure since 2013. Engineering, 21, 135–142.
https://doi.org/10.1016/j.eng.2022.03.014
Rakocinski, C.F., Menke, D.P., 2016.
Seasonal hypoxia regulates
macrobenthic function and structure in the Mississippi Bight. Mar. Pollut.
Bull. 105, 299–309.
https://doi.org/10.1016/j.marpolbul.2016.02.006
Rhoads, D.C., Boesch, D.F., Zhican, T., Fengshan, X., Liqiang, H., Nilsen,
K.J., 1985.
Macrobenthos and sedimentary facies on the Changjiang delta
platform and adjacent continental shelf, East China Sea. Cont. Shelf Res.
4, 189–213.
https://doi.org/10.1016/0278-4343(85)90029-9
Rouse, G.W., Pleijel, F., 2001.
Polychaetes. Oxford University Press,
Oxford.
Salmi, T., Maatta, A., Anttila, P., Ruoho-Airola, T., Amnell, T., 2002.
Detecting Trends of Annual Values of Atmospheric Pollutants by the
Mann-Kendall Test and Sen’s Solpe Estimates the Excel Template Application
MAKESENS. Air Quality Research, Finnish Meteorological Institute,
Helsinki.
Sang, Y.F., Fu, Q., Singh, V.P., Sivakumar, B., Zhu, Y., Li, X., 2020.
Does
summer precipitation in China exhibit significant periodicities? J.
Hydrol. 581, 124289.
https://doi.org/10.1016/j.jhydrol.2019.124289
Shi, Y., Zhang, Guicheng, Zhang, Guodong, Wen, Y., Guo, Y., Peng, L., Xu, W.,
Sun, J., 2022.
Species and functional diversity of marine macrobenthic
community and benthic habitat quality assessment in semi-enclosed waters upon
recovering from eutrophication, Bohai Bay, China. Mar. Pollut. Bull. 181.
https://doi.org/10.1016/j.marpolbul.2022.113918
Shou, L., Zeng, J., Liao, Y., Xu, T., Gao, A., Chen, Z., Chen, Q., Yang, J.,
2013.
Temporal and spatial variability of benthic macrofauna communities in
the Yangtze River estuary and adjacent area. Aquat. Ecosyst. Heal. Manag.
16, 31–39.
https://doi.org/10.1080/14634988.2013.759497
Silva, R.F., Rosa Filho, J.S., Souza, S.R., Souza-Filho, P.W., 2011.
Spatial and temporal changes in the structure of soft-bottom benthic
communities in an Amazon estuary (Caeté estuary, Brazil). J. Coast. Res.
440–444. h
ttps://doi.org/10.1007/s13131-024-0001-9
Su, J., Yuan, Y., 2005.
Coastal Hydrology of China. Ocean Press,
Beijing.
Sugni, M., Mozzi, D., Barbaglio, A., Bonasoro, F., Candia Carnevali, M.D.,
2007.
Endocrine disrupting compounds and echinoderms: New ecotoxicological
sentinels for the marine ecosystem. Ecotoxicology 16, 95–108.
https://doi.org/10.1007/s10646-006-0119-8
Sun, X., Fan, D., Liu, M., Liao, H., Tian, Y., 2019.
Persistent impact of
human activities on trace metals in the Yangtze River Estuary and the East
China Sea: Evidence from sedimentary records of the last 60 years. Sci.
Total Environ. 654, 878–889.
https://doi.org/10.1016/j.scitotenv.2018.10.439
Tang, Y., Liu, Q., Liao, Y., Zhou, K., Shou, L., 2021.
Responses of
macrobenthic communities to patchy distributions of heavy metals and petroleum
hydrocarbons in sediments: A study in China’s Zhoushan Archipelago. Acta
Oceanol. Sin. 40, 117–125.
https://doi.org/10.1007/s13131-021-1892-1
Teh, L.S.L., Cashion, T., Cheung, W.W.L., Sumaila, U.R., 2020.
Taking
stock: a Large Marine Ecosystem perspective of socio-economic and ecological
trends in East China Sea fisheries. Rev. Fish Biol. Fish. 30, 269–292.
https://doi.org/10.1007/s11160-020-09599-8
Tian, J., Zhang, W., Wu, J., Chen, Q., Huang, J., 2025.
Effects of hypoxia
on community structure of macrobenthos in the Pearl River Estuary. Acta
Oceanol. Sin. 44, 1–10.
https://doi.org/10.1007/s13131-024-0001-9
Wang, B., Chen, J., Jin, H., Li, H., Huang, D., Cai, W.J., 2017.
Diatom
bloom-derived bottom water hypoxia off the Changjiang estuary, with and without
typhoon influence. Limnol. Oceanogr. 62, 1552–1569.
https://doi.org/10.1002/lno.10517
Wang, K., Cai, W.J., Chen, J., Kirchman, D., Wang, B., Fan, W., Huang, D.,
2021.
Climate and human-driven variability of summer hypoxia on a large
river-dominated shelf as revealed by a hypoxia index. Front. Mar. Sci. 8,
1–12.
https://doi.org/10.3389/fmars.2021.634184
Wang, Z., Leung, K.M.Y., Sung, Y.H., Dudgeon, D., Qiu, J.W., 2021.
Recovery
of tropical marine benthos after a trawl ban demonstrates linkage between
abiotic and biotic changes. Commun. Biol. 4, 1–8.
https://doi.org/10.1038/s42003-021-01732-y
Warren, L.M., 1981.
Respiratory adaptations to temporary hypoxia by the
polychaete Cirriformia tentaculata. Comp. Biochem. Physiol. Pt. A, 69,
321–324.
https://doi.org/10.1016/0300-9629(81)90300-5
Xu, L., Song, P., Wang, Y., Xie, B., Huang, L., Li, Y., Zheng, X., Lin, L.,
2022.
Estimating the impact of a seasonal fishing moratorium on the East
China Sea ecosystem from 1997 to 2018. Front. Mar. Sci. 9, 1–15.
https://doi.org/10.3389/fmars.2022.865645
Xu, P., Peng, G., Su, L., Gao, Y., Gao, L., Li, D., 2018.
Microplastic risk
assessment in surface waters: A case study in the Changjiang Estuary,
China. Mar. Pollut. Bull. 133, 647–654.
https://doi.org/10.1016/j.marpolbul.2018.06.020
Xu, Y., Ma, L., Sui, J., Li, X., Wang, H., Zhang, B., 2022.
Potential
effects of climate change on the habitat suitability of macrobenthos in the
Yellow Sea and East China Sea. Mar. Pollut. Bull. 174, 113238.
https://doi.org/10.1016/j.marpolbul.2021.113238
Xu, Z.X., Li, J.Y., Takeuchi, K., Ishidaira, H., 2007.
Long-term trend of
precipitation in China and its association with the El Niño-southern
oscillation. Hydrol. Process. 21, 61–71.
https://doi.org/10.1002/hyp.6180
Xuan, F., Guan, W., Bao, C., Tang, F., Tang, B., Zhou, C., 2014.
Current
fishing practices may induce low risk of sperm limitation in female swimming
crab Portunus trituberculatus in the East China Sea. Aquat. Biol. 20,
145–153.
https://doi.org/10.3354/ab00555
Yan, J., Sui, J., Xu, Y., Li, X., Wang, H., Zhang, B., 2020.
Assessment of
the benthic ecological status in adjacent areas of the Yangtze River Estuary,
China, using AMBI, M-AMBI and BOPA biotic indices. Mar. Pollut. Bull. 153,
111020.
https://doi.org/10.1016/j.marpolbul.2020.111020
Yan, J., Xu, Y., Sui, J., Li, X., Wang, H., Zhang, B., 2017.
Longterm
variation of the macrobenthic community and its relationship with environmental
factors in the Yangtze River estuary and its adjacent area. Mar. Pollut.
Bull. 123, 339–348.
https://doi.org/10.1016/j.marpolbul.2017.09.023
Yang, H.F., Yang, S.L., Xu, K.H., Milliman, J.D., Wang, H., Yang, Z., Chen, Z.,
Zhang, C.Y., 2018.
Human impacts on sediment in the Yangtze River: A review
and new perspectives. Glob. Planet. Change 162, 8–17.
https://doi.org/10.1016/j.gloplacha.2018.01.001
Yu, W., Wen, J., Chen, X., Li, G., Li, Y., Zhang, Z., 2021.
Effects of
climate variability on habitat range and distribution of chub mackerel in the
East China Sea. J. Ocean Univ. China 20, 1483–1494. h
ttps://doi.org/10.1007/s11802-021-4760-x
Zhang, J., Pei, Z., He, P., Shi, J., 2021.
Effect of escape vents on
retention and size selectivity of crab pots for swimming crab Portunus
trituberculatus in the East China Sea. Aquac. Fish. 6, 340–347.
https://doi.org/10.1016/j.aaf.2020.04.008
Zhang, W., Dong, X., Liu, Z., Lin, R., Luo, H., 2021.
Influence of decadal
ocean signals on meteorological conditions associated with the winter haze over
Eastern China. Front. Environ. Sci. 9, 1–13.
https://doi.org/10.3389/fenvs.2021.727180
Zhao, H., Cao, Z., Liu, X., Zhan, Y., Zhang, J., Xiao, X., Yang, Y., Zhou, J.,
Xu, J., 2017.
Seasonal variation, flux estimation, and source analysis of
dissolved emerging organic contaminants in the Yangtze Estuary, China.
Mar. Pollut. Bull. 125, 208–215.
https://doi.org/10.1016/j.marpolbul.2017.08.034
Zheng, W., Song, M., Wang, L., Zhang, W., Li, Z., Zhu, L., Xie, W., Liang, Z.,
Jiang, Z., 2025.
Improving costal marine habitats in the northern yellow
sea: The role of artificial reefs on macrobenthic communities and
eco-exergy. Sci. Total Environ. 971, 179027.
https://doi.org/10.1016/j.scitotenv.2025.179027
Zhu, J., Wu, H., Li, L., Qiu, C., 2018. 4 Changjiang Estuary. [In:] Wang, X.
(Ed.),
Sediment Dynamics of Chinese Muddy Coasts and Estuaries: Physics,
Biology and Their Interactions. Acad. Press, London, 51–75.
https://doi.org/10.1016/B978-0-12-811977-8.00004-2
Zhu, Z.Y., Wu, Y., Zhang, J., Du, J.Z., Zhang, G. Sen, 2014.
Reconstruction
of anthropogenic eutrophication in the region off the Changjiang Estuary and
central Yellow Sea: From decades to centuries. Cont. Shelf Res. 72,
152–162.
https://doi.org/10.1016/j.csr.2013.10.018
Long-term variability of sound speed conditions in Hornsund fjord, Svalbard, between 2001 and 2019
Oceanologia, 67 (4)/2025, 67406, 18 pp.
https://doi.org/10.5697/GRSC5449
Pavani Vithana Madugeta Vidanamesthrige*,1, Natalia Gorska1, Oskar Głowacki2
1Institute of Oceanology, Polish Academy of Sciences, Powstańców Warszawy 55, 81-712 Sopot, Poland;
e-mail: pavani@iopan.pl (P. V. Madugeta Vidanamesthrige)
2Institute of Geophysics, Polish Academy of Sciences, Księcia Janusza 64, 01-452 Warszawa, Poland
*corresponding author
Keywords:
Hornsund; Glacier melting; Shelf-fjord water exchange; Underwater sound; Marine mammals
Received: 28 October 2024; revised: 19 August 2025; accepted: 16 September 2025
Highlights
- Sound speed conditions changed in Hornsund in 2001–2019 under climate warming.
- Understanding of the mechanism controlling variation of sound speed conditions was improved.
- Climate-driven fjord Atlantification prevented deep sound channel formation recently.
- Recently, near-surface channels have become more pronounced due to accelerated glacial retreat.
- Future changes in sound pollution and marine animals’ well-being.
Abstract
The glacierised Arctic fjords are particularly sensitive to oceanic and atmospheric warming caused by climate shifts;
the melting of glaciers and icebergs is one of the major indicators of this sensitivity. The meltwater delivery to the
ocean changes the thermohaline structure of the water column, which not only affects water mixing but also controls
the sound speed conditions; the latter is crucial for the variability of underwater sound propagation. Finally, changes
in sound propagation conditions affect marine animals that rely on sound for their key biological functions, such as
communication, navigation, and mating. Here, we investigate the long-term variability of sound speed conditions in
the Hornsund fjord, Svalbard, together with its governing factors. We calculated the vertical sound speed profiles using
temperature and salinity data collected along the fjord centerline from 2001 to 2019. Spatial and temporal variability
in sound speed conditions are observed. We identify two major types of sound channels: (i) near-surface sound channel
and (ii) deep sound channel. Potential physical mechanisms that govern the presence and position of these sound
channels are discussed, including glacier melting, shelf-fjord water exchange, and atmospheric heat flux. In recent
years, the climate-driven transformation of Hornsund has led to the disappearance of deep sound channels due to the
intensified inflow of Atlantic Water, and an increased presence and extent of near-surface sound channels resulting
from elevated freshwater input from melting glaciers. We suggest that climate warming-induced changes in sound
speed conditions in Hornsund are likely to have far-reaching consequences for underwater sound pollution, potentially
impacting the well-being of marine animals
References 
Affatati, A., Scaini, C., Salon, S., 2022.
Ocean Sound Propagation in a
Changing Climate: Global Sound Speed Changes and Identification of Acoustic
Hotspots. Earth’s Future 10(3).
https://doi.org/10.1029/2021ef002099
Alexander, P., Duncan, A., Bose, N., Williams, G., 2016.
Modelling acoustic
propagation beneath Antarctic sea ice using measured environmental
parameters. Deep Sea Res. Pt. II. 131, 84–95.
http://dx.doi.org/10.1016/j.dsr2.2016.04.026
Arntsen, M., Sundfjord, A., Skogseth, R., Błaszczyk, M., Promińska, A.,
2019.
Inflow of Warm Water to the Inner Hornsund Fjord, Svalbard: Exchange
Mechanisms and Influence on Local Sea Ice Cover and Glacier Front Melting.
J. Geophys. Res: Oceans. 124, 1915–1931.
https://doi.org/10.1029/2018jc014315
Årthun, M., Eldevik, T., Smedsrud, L.H., 2019.
The Role of Atlantic Heat
Transport in Future Arctic Winter Sea Ice Loss. J. Clim. 32, 3327–3341.
https://doi.org/10.1175/jcli-d-18-0750.1
Bagočius, D., 2015.
Potential Masking of the Baltic Grey Seal
Vocalisations by Underwater Shipping Noise in the Lithuanian Area of the Baltic
Sea. EREM 4(70), 66–72.
https://doi.org/10.5755/j01.erem.70.4.6913
Baggeroer, A. B., Collis, J. M., 2022.
Transmission loss for the Beaufort
Lens and the critica frequency for mode propagation during ICEX-18. J.
Acoust. Soc. Am. 151(4), 2760–2772.
https://doi.org/10.1121/10.0010049
Ballard, M. S., 2019.
Three-dimensional acoustic propagation effects
induced by the sea ice canopy. J. Acoust. Soc. Am. 146(4), EL364–EL368.
https://doi.org/10.1121/1.5129554
Ballard, M. S., Badiey, M., Sagers, J. D., Colosi, J. A., Turgut, A., Pecknold,
S., Lin, Y.-T., Proshutinsky, A., Krishfield, R., Worcester, P. F., 2020.
Temporal and spatial dependence of a yearlong record of sound propagation
from the Canada Basin to the Chukchi Shelf. J. Acoust. Soc. Am. 148(3),
1663–1680.
https://doi.org/10.1121/10.0001970
Bengtsson, O., Lydersen, C., Kovacs, K. M., 2022.
Cetacean spatial trends
from 2005 to 2019 in Svalbard, Norway. Polar Res. 41.
http://dx.doi.org/10.33265/polar.v41.7773
Błaszczyk, M., Ignatiuk, D., Uszczyk, A., Cielecka-Nowak, K., Grabiec, M.,
Jania, J.A., Moskalik, M., Walczowski, W., 2019.
Freshwater input to the
Arctic fjord Hornsund (Svalbard). Polar Res. 38.
https://doi.org/10.33265/polar.v38.3506
Błaszczyk, M., Jania, J.A., Kolondra, L., 2013.
Fluctuations of tidewater
glaciers in Hornsund Fjord (Southern Svalbard) since the beginning of the 20th
century. Pol. Polar Res. 34, 327–352.
https://doi.org/10.2478/popore-2013-0024
Błaszczyk, M., Moskalik, M., Grabiec, M., Jania, J., Walczowski, W.,
Wawrzyniak, T., Strzelewicz, A., Malnes, E., Lauknes, T.R., Pfeffer, W.T.,
2023.
The Response of Tidewater Glacier Termini Positions in Hornsund
(Svalbard) to Climate Forcing, 1992–2020. J. Geophys. Res. Earth Surf.
128.
https://doi.org/10.1029/2022jf006911
Chauché, N., Hubbard, A., Gascard, J.C., Box, J.E., Bates, R., Koppes, M.,
Sole, A., Christoffersen, P., Patton, H., 2014.
Ice–ocean interaction and
calving front morphology at two west Greenland tidewater outlet glaciers.
Cryosphere 8, 1457–1468.
https://doi.org/10.5194/tc-8-1457-2014
Chen, C.-T., Millero, F.J., 1977.
Sound speed in seawater at high
pressures. J. Acoust. Soc. Am. 62, 1129–1135.
https://doi.org/10.1121/1.381646
Chitre, M., Shahabudeen, S., Stojanovic, M., 2008.
Underwater acoustic
communications and networking: Recent advances and future challenges. Mar.
Technol. Soc. J. 42, 103–116.
https://doi.org/10.4031/002533208786861263
Cook, A.J., Copland, L., Noël, B.P., Stokes, C.R., Bentley, M.J., Sharp,
M.J., Bingham, R.G., van den Broeke, M.R., 2019.
Atmospheric forcing of
rapid marine-terminating glacier retreat in the Canadian Arctic
Archipelago. Sci. Adv. 5.
https://doi.org/10.1126/sciadv.aau8507
Dahlke, S., Hughes, N.E., Wagner, P.M., Gerland, S., Wawrzyniak, T., Ivanov,
B., Maturilli, M., 2020.
The observed recent surface air temperature
development across Svalbard and concurring footprints in local sea ice
cover. Int. J. Climatol. 40, 5246–5265.
https://doi.org/10.1002/joc.6517
De Rovere, F., Langone, L., Schroeder, K., Miserocchi, S., Giglio, F., Aliani,
S., Chiggiato, J., 2022.
Water Masses Variability in Inner Kongsfjorden
(Svalbard) During 2010–2020. Front. Mar. Sci. 9.
https://doi.org/10.3389/fmars.2022.741075
Deane, G.B., Glowacki, O., Stokes, D., Pettit. E., 2019.
The Underwater
Sounds of Glaciers. Acoust. Today. 15, 12.
https://doi.org/10.1121/at.2019.15.4.12
Deane, G.B., Glowacki, O., Tegowski, J., Moskalik, M., Blondel, P., 2014.
Directionality of the ambient noise field in an Arctic, glacial bay.
J. Acoust. Soc. Am. 136, EL350-6. https://doi.org/10.1121/1.4897354
Diachok, O. I., 1976. Effects of sea-ice ridges on sound propagation in the
Arctic Ocean. J. Acoust. Soc. Am. 59(5), 1110–1120. https://doi.org/10.1121/1.380965
Duarte, C.M., Chapuis, L., Collin, S.P., Costa, D.P., Devassy, R.P., Eguiluz,
V.M., Erbe, C., Gordon, T.A.C., Halpern, B.S., Harding, H.R., Havlik, M.N.,
Meekan, M., Merchant, N.D., Miksis-Olds, J.L., Parsons, M., Predragovic, M.,
Radford, A.N., Radford, C.A., Simpson, S.D., Slabbekoorn, H., Staaterman, E.,
Van Opzeeland, I.C., Winderen, J., Zhang, X., Juanes, F., 2021. The
soundscape of the Anthropocene ocean. Science 371. https://doi.org/10.1126/science.aba4658
Duda, T. F., 2017. Acoustic signal and noise changes in the Beaufort Sea
Pacific Water duct under anticipated future acidification of Arctic Ocean
waters. J. Acoust. Soc. Am. 142(4), 1926–1933. https://doi.org/10.1121/1.5006184
Duda, T. F., Zhang, W. G., Lin, Y. T., 2021. Effects of Pacific Summer
Water layer variations and ice cover on Beaufort Sea underwater sound
ducting. J. Acoust. Soc. Am. 149(4), 2117–2136. https://doi.org/10.1121/10.0003929
Erbe, C., Dunlop, R., Dolman, S., 2018. Effects of Noise on Marine
Mammals. [in:] Slabbekoorn, H., Dooling, R.J., Popper, A.N., Fay, R.R.
(Eds.), Effects of Anthropogenic Noise on Animals. Springer Handbook
of Auditory Research, 277–309. https://doi.org/10.1007/978-1-4939-8574-6
Erbe, C., Farmer, D. M., 2000. Zones of impact around icebreakers affecting
beluga whales in the Beaufort Sea. J. Acoust. Soc. Am. 108(3),
1332–1340. https://doi.org/10.1121/1.1288938
Erbe, C., Marley, S.A., Schoeman, R.P., Smith, J.N., Trigg, L.E., Embling,
C.B., 2019. The Effects of Ship Noise on Marine Mammals — A Review.
Front. Mar. Sci. 6. https://doi.org/10.3389/fmars.2019.00606
Erbe, C., Reichmuth, C., Cunningham, K., Lucke, K., Dooling, R., 2016.
Communication masking in marine mammals: A review and research
strategy. Mar. Pollut. Bull. 103, 15–38. https://doi.org/10.1016/j.marpolbul.2015.12.007
Geyman, E.C., W, J.J.v.P., Maloof, A.C., Aas, H.F., Kohler, J., 2022.
Historical glacier change on Svalbard predicts doubling of mass loss by
2100. Nature. 601, 374–379. https://doi.org/10.1038/s41586-021-04314-4
Gjelten, H.M., Nordli, Ø., Isaksen, K., Førland, E.J., Sviashchennikov, P.N.,
Wyszynski, P., Prokhorova, U.V., Przybylak, R., Ivanov, B.V., Urazgildeeva,
A.V., 2016. Air temperature variations and gradients along the coast and
fjords of western Spitsbergen. Polar Res. 35, 29878. https://doi.org/10.3402/polar.v35.29878
Glowacki, O., 2020. Underwater noise from glacier calving: Field
observations and pool experiment. J. Acoust. Soc. Am. 148, EL1–EL7. https://doi.org/10.1121/10.0001494
Glowacki, O., Deane, G.B., Moskalik, M., 2018. The Intensity,
Directionality, and Statistics of Underwater Noise From Melting Icebergs.
Geophys. Res. Lett. 45, 4105–4113. https://doi.org/10.1029/2018gl077632
Glowacki, O., Deane, G.B., Moskalik, M., Blondel, P., Tegowski, J., Blaszczyk,
M., 2015. Underwater acoustic signatures of glacier calving. Geophys.
Res. Lett. 42, 804–812. https://doi.org/10.1002/2014gl062859
Glowacki, O., Moskalik, M., Deane, G.B., 2016. The impact of glacier
meltwater on the underwater noise field in a glacial bay. J. Geophys. Res:
Oceans. 121, 8455–8470. https://doi.org/10.1002/2016jc012355
Glowacki, O., Moskalik, M., Prominska, A., 2013. Simulation of the sound
propagation in an Arctic fjord: general patterns and variability. poster
presented at Arctic Science Summit Week, Committee on Polar Research of the
Polish Academy of Sciences, Cracow, Poland.
Halliday, W. D., Insley, S. J., Hilliard, R. C., de Jong, T. Pine, M. K., 2017.
Potential impacts of shipping noise on marine mammals in the western
Canadian Arctic. Mar. Pollut. Bull. 123(1–2), 73–82. http://dx.doi.org/10.1016/j.marpolbul.2017.09.027
Halliday, W. D., Pine, M. K. Insley, S. J., 2020. Underwater noise and
Arctic marine mammals: Review and policy recommendations. Environ. Rev.
28(4), 438–448. https://doi.org/10.1139/er-2019-003
Halliday, W. D., Scharffenberg, K., MacPhee, S., Hilliard, R. C., Mouy, X.,
Whalen, D., Loseto, L. L. Insley, S. J., 2019. Beluga Vocalizations
Decrease in Response to Vessel Traffic in the Mackenzie River Estuary.
Arctic. 72(4), 337–346. https://doi.org/10.14430/arctic69294
Holmes, F.A., Kirchner, N., Kuttenkeuler, J., Krutzfeldt, J., Noormets, R.,
2019. Relating ocean temperatures to frontal ablation rates at Svalbard
tidewater glaciers: Insights from glacier proximal datasets. Sci. Rep. 9,
9442. https://doi.org/10.1038/s41598-019-45077-3
Hopkins, T.S., 1991. The GIN Sea—A synthesis of its physical oceanography
and literature review 1972–1985. Earth-Sci. Rev. 30, 175–318. https://doi.org/10.1016/0012-8252(91)90001-V
IPCC 2021. Climate change 2021: The physical science basis.
Contribution of working group I to the sixth assessment report of the
intergovernmental panel on climate change. Jain, V., Korhonen, M., Głowacki,
O. Moskalik, M., 2024. Hydrography of the Inner Basins in Hornsund (Svalbard):
Heat Advection Near Tidewater Glaciers. J. Geophys. Res.: Oceans, 129(11). https://doi.org/10.1029/2024JC021273
Jakacki, J., Przyborska, A., Kosecki, S., Sundfjord, A., Albretsen, J., 2017.
Modelling of the Svalbard fjord Hornsund. Oceanologia 59(4),
473–495. https://doi.org/10.1016/j.oceano.2017.04.004
Jensen, F.B., Kuperman, W.A., Porter, M.B., Schmidt, H., Tolstoy, A., 2011.
Computational ocean acoustics. 2nd edn., Springer, New York.
Korhonen, M., Moskalik, M., Głowacki, O., Jain, V., 2024. Oceanographic
monitoring in Hornsund fjord, Svalbard. Earth Syst. Sci. Data. 16(10),
4511–4527. https://doi.org/10.5194/essd-16-4511-2024
Kucukosmanoglu, M., Colosi, J. A., Worcester, P. F., Dzieciuch, M. A., Sagen,
H., Duda, T. F., Zhang, W. G., Miller, C. W., Richards, E. L., 2023.
Observations of the space/time scales of Beaufort sea acoustic duct
variability and their impact on transmission loss via the mode interaction
parameter. J. Acoust. Soc. Am. 153(5), 2659–2676. https://doi.org/10.1121/10.0019335
Kutschale, H., 1969. Arctic hydroacoustics. Arctic. 22, 169–364. https://doi.org/10.14430/arctic3218
Kystdatahuset, 2025. Tall og statistikk-Trafikk i område. https://kystdatahuset.no/tallogstatistikk/trafikkiom
rade (Accessed 01.07.2025).
Lind, S., Ingvaldsen, R.B., Furevik, T., 2018. Arctic warming hotspot in
the northern Barents Sea linked to declining sea-ice import. Nat. Clim.
Change. 8, 634–639. https://doi.org/10.1038/s41558-018-0205-y
Luckman, A., Benn, D.I., Cottier, F., Bevan, S., Nilsen, F., Inall, M., 2015.
Calving rates at tidewater glaciers vary strongly with ocean
temperature. Nat. Commun. 6, 8566. https://doi.org/10.1038/ncomms9566
Lyamin, O. I., Korneva, S. M., Rozhnov, V. V., Mukhametov, L. M., 2011.
Cardiorespiratory changes in beluga in response to acoustic noise.
Dokl. Biol. Sci. 440(5), 275–278. https://doi.org/10.1134/S0012496611050218
Lynch, J., Gawarkiewicz, G., Lin, Y.-T., Duda, T., Newhall, A., 2018.
Impacts of Ocean Warming on Acoustic Propagation Over Continental Shelf and
Slope Regions. Oceanography 31(2), 174–181. https://doi.org/10.5670/oceanog.2018.219
Marsz, A.A., Styszyńska, A., 2013. Climate and climate change at
Hornsund, Svalbard. Gdynia Marit. Univ., Gdynia, ISBN: 978-83-7421-191-8.
Martin, M. J., Halliday, W. D., Storrie, L., Citta, J. J., Dawson, J., Hussey,
N. E., Juanes, F., Loseto, L. L., MacPhee, S. A., Moore, L., Nicoll, A.,
O’Corry-Crowe, G., Insley, S. J., 2022. Exposure and behavioral responses
of tagged beluga whales (Delphinapterus leucas) to ships in the Pacific
Arctic. Mar. Mamm. Sci. 39(2), 387–421. https://doi.org/10.1111/mms.12978
Matsumoto, H., Bohnenstiehl, D.R., Tournadre, J., Dziak, R.P., Haxel, J.H.,
Lau, T.K.A., Fowler, M., Salo, S.A., 2014. Antarctic icebergs: A
significant natural ocean sound source in the Southern Hemisphere.
Geochem. Geophys. 15, 3448–3458. https://doi.org/10.1002/2014gc005454
McCammon, D. F., McDaniel, S. T., 1985. The influence of the physical
properties of ice on reflectivity. J. Acoust. Soc. Am. 77(2), 499–507.
https://doi.org/10.1121/1.391869
Medwin, H., Clay, C.S., 1998. Fundamentals of acoustical oceanography.
Acad. Press, New York.
Meyer, A., Eliseev, D., Heinen, D., Linder, P., Scholz, F., Weinstock, L. S.,
Wiebusch, C., Zierke, S., 2019. Attenuation of sound in glacier ice from 2
to 35 kHz. The Cryosphere. 13(4), 1381–1394. https://doi.org/10.5194/tc-13-1381-2019
Moskalik, M., Grabowiecki, P., Tęgowski, J., Żulichowska, M., 2013.
Bathymetry and geographical regionalization of Brepollen (Hornsund,
Spitsbergen) based on bathymetric profiles interpolations. Pol. Polar Res.
34, 1–22. https://doi.org/10.2478/popore−2013−0001
Munk, W., Worcester, P., Wunsch, C., 1995. Ocean acoustic tomography.
Cambridge Univ. Press, Cambridge, England.
Nilsen, F., Cottier, F., Skogseth, R., Mattsson, S., 2008. Fjord–shelf
exchanges controlled by ice and brine production: The interannual variation of
Atlantic Water in Isfjorden, Svalbard. Cont. Shelf Res. 28, 1838–1853.
https://doi.org/10.1016/j.csr.2008.04.015
Nilsen, F., Skogseth, R., Vaardal-Lunde, J., Inall, M., 2016. A Simple
Shelf Circulation Model: Intrusion of Atlantic Water on the West Spitsbergen
Shelf. J. Phys. Oceanogr. 46, 1209–1230. https://doi.org/10.1175/jpo-d-15-0058.1
Overland, J.E., Wang, M., Walsh, J.E., Stroeve, J.C., 2013. Future Arctic
climate changes: Adaptation and mitigation time scales. Earth’s Future.
2, 68–74. https://doi.org/10.1002/2013ef000162
PAME 2019. Underwater noise in the Arctic: A state of knowledge
report. Protection of the Arctic Marine Environment (PAME) International
Secretariat.
PAME, 2025. PAME, Arctic Shipping Status Rep.no 1. https://hdl.handle.net/11374/2733.3
Pettit, E.C., Lee, K.M., Brann, J.P., Nystuen, J.A., Wilson, P.S., O’Neel,
S., 2015. Unusually loud ambient noise in tidewater glacier fjords: A
signal of ice melt. Geophys. Res. Lett. 42, 2309–2316. https://doi.org/10.1002/2014GL062950
Podolskiy, E.A., Murai, Y., Kanna, N., Sugiyama, S., 2022. Glacial
earthquake-generating iceberg calving in a narwhal summering ground: The
loudest underwater sound in the Arctic? J. Acoust. Soc. Am. 151(1),
6–16. https://doi.org/10.1121/10.0009166
Polyakov, I.V., Pnyushkov, A.V., Alkire, M.B., Ashik, I.M., Baumann, T.M.,
Carmack, E.C., Goszczko, I., Guthrie, J., Ivanov, V.V., Kanzow, T., 2017.
Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of
the Arctic Ocean. Science. 356, 285–291. https://doi.org/10.1126/science.aai8204
Popov, V. V., Supin, A. Y., Rozhnov, V. V., Nechaev, D. I., Sysuyeva, E. V.,
Klishin, V. O., Pletenko, M. G., Tarakanov, M. B., 2013. Hearing threshold
shifts and recovery after noise exposure in beluga whales, Delphinapterus
leucas. J. Exp. Biol. 216(9), 1587–1596. https://doi.org/1587-1596,10.1242/jeb.078345
Possenti, L., Reichart, G. J., de Nooijer, L., Lam, F. P., de Jong, C., Colin,
M., Binnerts, B., Boot, A., von der Heydt, A., 2023. Predicting the
contribution of climate change on North Atlantic underwater sound
propagation. PeerJ, 11 pp . https://doi.org/10.7717/peerj.16208
Promińska, A., Cisek, M., Walczowski, W., 2017. Kongsfjorden and Hornsund
hydrography – comparative study based on a multiyear survey in fjords of west
Spitsbergen. Oceanologia 59(4), 397–412. https://doi.org/10.1016/j.oceano.2017.07.003
Promińska, A., Falck, E., Walczowski, W., 2018. Interannual variability
in hydrography and water mass distribution in Hornsund, an Arctic fjord in
Svalbard. Polar Res. 37, 1495546. https://doi.org/10.1080/17518369.2018.1495546
Rodriguez, J. P., Klemm, K., Duarte, C. M., Eguiluz, V. M., 2024. Shipping
traffic through the Arctic Ocean: Spatial distribution, seasonal variation, and
its dependence on the sea ice extent. iScience 27(7), 110236. https://doi.org/10.1016/j.isci.2024.110236
Shu, Q., Wang, Q., Årthun, M., Wang, S., Song, Z., Zhang, M., Qiao, F., 2022.
Arctic Ocean Amplification in a warming climate in CMIP6 models. Sci.
Adv. 8(30). https://doi.org/10.1126/sciadv.abn9755
Skogseth, R., Olivier, L.L.A., Nilsen, F., Falck, E., Fraser, N., Tverberg, V.,
Ledang, A.B., Vader, A., Jonassen, M.O., Søreide, J., Cottier, F., Berge, J.,
Ivanov, B.V., Falk-Petersen, S., 2020. Variability and decadal trends in
the Isfjorden (Svalbard) ocean climate and circulation – An indicator for
climate change in the European Arctic. Prog. Oceanogr. 187. https://doi.org/10.1016/j.pocean.2020.102394
Slater, D.A., Straneo, F., 2022. Submarine melting of glaciers in Greenland
amplified by atmospheric warming. Nat. Geosci. 15, 794–799. https://doi.org/10.1038/s41561-022-01035-9
Storheim, E., Sagen, H., Dzieciuch, M. A., Worcester, P. F., 2022.
Modelling of sound propagation across the Arctic Ocean using oceanographic
fields and oceanographic data. J. Acoust. Soc. Am. 152(4). https://doi.org/10.1121/10.0010047
Straneo, F., Curry, R.G., Sutherland, D.A., Hamilton, G.S., Cenedese, C.,
Våge, K., Stearns, L.A., 2011. Impact of fjord dynamics and glacial
runoff on the circulation near Helheim Glacier. Nat. Geosci. 4, 322–327.
https://doi.org/10.1038/ngeo1109
Strzelewicz, A., Przyborska, A., Walczowski, W., 2022. Increased presence
of Atlantic Water on the shelf southwest of Spitsbergen with implications for
the Arctic fjord Hornsund. Prog. Oceanogr. 200, 102714. https://doi.org/10.1016/j.pocean.2021.102714
Tverberg, V., Skogseth, R., Cottier, F., Sundfjord, A., Walczowski, W., Inall,
M.E., Falck, E., Pavlova, O., Nilsen, F., 2019. The Kongsfjorden transect:
seasonal and interannual variability in hydrography. [in:] Haakon, H.,
Christian, W. (Eds.), The Ecosystem of Kongsfjorden, Svalbard. Springer, Cham.
2, 49–104. https://doi.org/10.1007/978-3-319-46425-1_3
Urick, R.J., 1971. The noise of melting icebergs. J. Acoust. Soc. Am.
50, 337–341. https://doi.org/10.1121/1.1912637
Urick, R.J., 1979. Sound propagation in the sea. Defence Adv. Res. Project
Agency, Washington, D. C. van Pelt, W., Pohjola, V., Pettersson, R., Marchenko,
S., Kohler, J., Luks, B., Hagen, J.O., Schuler, T.V., Dunse, T., Noël, B.,
Reijmer, C., 2019. A long-term dataset of climatic mass balance, snow
conditions, and runoff in Svalbard (1957–2018). Cryosphere. 13,
2259-02280. https://doi.org/10.5194/tc-13-2259-2019
van Pelt, W.J.J., Schuler, T.V., Pohjola, V.A., Pettersson, R., 2021.
Accelerating future mass loss of Svalbard glaciers from a multi-model
ensemble. J. Glaciol. 67, 485–0499. https://doi.org/10.1017/jog.2021.2
Vishnu, H., Deane, G.B., Chitre, M., Glowacki, O., Stokes, D., Moskalik, M.,
2020. Vertical directionality and spatial coherence of the sound field in
glacial bays in Hornsund Fjord. J. Acoust. Soc. Am. 148, 3849–3862. https://doi.org/10.1121/10.0002868
Vishnu, H., Deane, G.B., Glowacki, O., Chitre, M., Johnson, H., Moskalik, M.,
Stokes, D., 2023. Depth-dependence of the underwater noise emission from
melting glacier ice. JASA Express Lett. 3, 020801-1-020801-7. https://doi.org/10.1121/10.0017348
Walczowski, W., Piechura, J., 2011. Influence of the West Spitsbergen
Current on the local climate. Int. J. Climatol. 31, 1088–1093. https://doi.org/10.1002/joc.2338
Wang, Q., Wekerle, C., Wang, X., Danilov, S., Koldunov, N., Sein, D.,
Sidorenko, D., von Appen, W.J., Jung, T., 2020. Intensification of the
Atlantic Water Supply to the Arctic Ocean Through the Fram Strait Induced by
Arctic Sea Ice Decline. Geophys. Res. Lett. 47(3), e2019GL086682. https://doi.org/10.1029/2019gl086682
Wawrzyniak, T., Osuch, M., 2020. A 40-year High Arctic climatological
dataset of the Polish Polar Station Hornsund (SW Spitsbergen, Svalbard).
Earth Syst. Sci. Data. 12, 805–815. https://doi.org/10.5194/essd-12-805-2020
Worcester, P. F., Badiey, M., Sagen, H., 2022. Introduction to the special
issue on ocean acoustics in the changing Arctic. J. Acoust. Soc. Am.
151(4), 2787–2790. https://doi.org/10.1121/10.0010308
Worcester, P. F., Dzieciuch, M. A., Sagen, H., 2020. Ocean Acoustics in the
Rapidly Changing Arctic. Acoust. Today. 16(1), 55–64. https://doi.org/10.1121/AT.2020.16.1.55
Zeh, M. C., Ballard, M. S., Glowacki, O., Deane, G. B., Wilson, P. S., 2022.
Model-data comparison of sound propagation in a glacierised fjord with a
simulated brash ice surface. J. Acoust. Soc. Am. 151(4), 2367–2377. https://doi.org/10.1121/10.0010046
Perspectives of seasonal hydrography and water masses in Saudi waters of the Arabian Gulf
Oceanologia, 67 (4)/2025, 67407, 16 pp.
https://doi.org/10.5697/KPCV6249
Mohamed Asharaf1, V.M. Aboobacker2,*, C.P. Abdulla2, Thadickal V. Joydas1, Karuppasamy P. Manikandan1, M. Rafeeq1, Abdulaziz Al-Suwailem1, P. Vethamony2
1Applied Research Center for Environment and Marine Studies, Research & Innovation, King Fahd University of Petroleum and Minerals, P. B. No. 391, Dhahran 31261, Saudi Arabia;
2Environmental Science Center, Qatar University, P.B. No. 2713, Doha, Qatar
e-mail: vmaboobacker@qu.edu.qa (V.M. Aboobacker)
*corresponding author
Keywords:
Seasonal hydrography; Intrusion of IOSW; Stratification; Vertical homogeneity; Arabian Gulf
Received: 2 June 2025; revised: 4 September 2025; accepted: 30 September 2025
Highlights
- CTD profiles from 555 stations along the Saudi coast of the Arabian Gulf were analyzed.
- Seasonal and spatial variability in hydrography were examined.
- The presence of three water masses was identified in Saudi waters.
- Hypersalinity in the shallow embayments along the Saudi coast was investigated.
- The extension of IOSW was identified in Saudi waters.
Abstract
The hydrographic characteristics and water mass features of the Saudi waters of the Arabian Gulf (Persian Gulf) are less documented compared to neighboring regions. This study analyzed vertical profiles of temperature, salinity, and density collected from five transects with 555 stations in Saudi waters during five seasons. Although the data were collected during 2002–2003, they reveal notable hydrographic variability and features associated with Saudi waters.
The sea surface temperature during late autumn and winter shows strong horizontal fluctuations between the northern and southern belts of the Saudi coast, while a high temperature plume is formed in the central coast during early summer. The central and northern coasts of Saudi Arabia have high concentrations of salinity induced by shallow embayments, while the impact of brine is limited to small areas in the vicinity of the outfalls. The presence of three water masses, namely, Indian Ocean Surface Water (IOSW), Arabian Gulf Water (AGW), and Bay Systems-induced Water (BSW), has been evident in this region; however, they co-occur only during spring, early summer, and summer in central and northern transects. The autumn and winter are characterized by the presence of AGW and BSW in all transects, while the IOSW was absent due to the mixing and by the opposing effects of shamal winds, which diminishes the inflow of IOSW. Nonetheless, the early summer and summer, with strong thermal stratification, exhibit the progression of IOSW up to the northern end of the Saudi waters.
References 
Aboobacker, V.M., Hasna, V.M., Al-Ansari, E.M.A.S., Vethamony, P., 2024.
Nearshore hydrography along the coast of Doha, central Arabian Gulf.
Coastal Res. 113 (Sp. Iss.), 422–426.
Aboobacker, V.M., Samiksha, S.V., Veerasingam, S., Al-Ansari, E.M.A.S.,
Vethamony, P., 2021c.
Role of shamal and easterly winds on the wave
characteristics off Qatar, Central Arabian Gulf. Ocean Eng. 236, 109457.
Aboobacker, V.M., Shanas, P.R., Al-Ansari, E.M.A.S., Sanil Kumar, V.,
Vethamony, P., 2021b.
The maxima in northerly wind speeds and wave heights
over the Arabian Sea, the Arabian/Persian Gulf and the Red Sea derived from 40
years of ERA5 data. Climate Dynam. 56, 1037–1052.
Aboobacker, V.M., Shanas, R.P, Veerasingam, S., Al-Ansari E.M.A.S., Sadooni,
F.N., Vethamony, P., 2021a.
Long-Term Assessment of Onshore and Offshore
Wind Energy Potentials of Qatar. Energies 14, 1178–1178.
Al-Abdulkader, K.A., Loughland, R.A., Qurban, M.A., 2019.
Ecosystems and
biodiversity of the Arabian Gulf. Saudi Arabian waters. In: Fifty Years of
Scientific Research. Publi. Saudi Aramco and King Fahd Univ. Petrol. Min.,
Dhahran, Saudi Arabia, 624 pp.
Al-Ansari, E.M., Husrevoglu, Y.S., Yigiterhan, O., Youssef, N., Al-Maslamani,
I.A., Abdel-Moati, M.A. Vethamony, P., 2022.
Seasonal variability of
hydrography off the east coast of Qatar, central Arabian Gulf. Arabian J.
Geosci. 15 (22), 1659.
https://doi.org/10.1007/s12517-022-10927-4
Al-Ansari, E.M., Rowe, G., Abdel-Moati, M.A.R., Yigiterhan, O., Al-Maslamani,
I., Al-Yafei, M.A., Al-Shaikh, I., Upstill- Goddard, R., 2015.
Hypoxia in
the central Arabian Gulf Exclusive Economic Zone (EEZ) of Qatar during summer
season. Estuar. Coast. Shelf Sci. 159, 60–68.
Alosairi, Y., Al-Salem, S.M., Al Ragum, A., 2020.
Threedimensional
numerical modelling of transport, fate and distribution of microplastics in the
northwestern Arabian/ Persian Gulf. Mar. Pollut. Bull. 161, 111723.
https://doi.org/10.1016/j.marpolbul.2020.111723
Alosairi, Y., Pokavanich, T., 2017.
Seasonal circulation assessments of the
northern Arabian/Persian Gulf. Mar. Pollut. Bull. 116 (1–2), 270–290.
Asharaf, M., Aboobacker, V.M., Abdulla, C.P., Joydas, T.V., Manikandan, K.,
Rafeeq, M., Al-Suwailem, A., Vethamony, P., 2025.
In situ Data of different
seasons from the Saudi waters of the Arabian Gulf. Figshare, dataset.
https://doi.org/10.6084/m9.figshare.29974675.v1
de Marez, C., L’Hégaret, P., Morvan, M., Carton, X., 2019.
On the 3D
structure of eddies in the Arabian Sea. Deep-Sea Res. Pt. I, 150, 103057.
Elobaid, E.A., Al-Ansari, E.M.A.S., Yigiterhan, O., Aboobacker, V.M.,
Vethamony, P., 2022.
Spatial variability of summer hydrography in the
central Arabian Gulf. Oceanologia, 64 (1), 75–87.
Ghaemi, M., Gholamreza, M., Samad, H., Sara, G., 2022.
Spatial and temporal
characterizations of seawater quality on marine waters area of the Persian
Gulf. Reg. Stud. Mar. Sci. 53, 102407.
https://doi.org/10.1016/j.rsma.2022.102407
Hanert, E., Aboobacker, V.M., Veerasingam, S., Dobbelaere, T., Vallaeys, V.,
Vethamony, P., 2023.
Multiscale ocean modelling system for the central
Arabian/Persian Gulf: From regional to structure scale circulation
patterns. Estuar. Coast. Shelf Sci. 282, 108230.
https://doi.org/10.1016/j.ecss.2023.108230
Hassanzadeh, S., Hosseinibalam, F., Rezaei-Latifi, A., 2011.
Numerical
modelling of salinity variations due to wind and thermohaline forcing in the
Persian Gulf. Appl. Math. Model. 35 (3), 1512–1537.
https://doi.org/10.1016/j.apm.2010.09.029
Hunter, J.R., 1986.
The physical oceanography of the Arabian Gulf: a review
and theoretical interpretation of previous observations. [In:] First Gulf
Conference on Environment and Pollution 7-9, February (1982), Kuwait, R. Halway
(Ed).
Hunter, J.R., 1984.
A review of the residual circulation and mixing process
in the KAP region. [In:] Oceanographic modelling of the Kuwait Action Plan
Region, M.I. El-Sabh (Ed.), UNESCO Rep. Mar. Sci., 28, 37–45.
Ibrahim, H.D., Xue, P., Eltahir, E.A., 2020.
Multiple salinity equilibria
and resilience of Persian/Arabian Gulf basin salinity to brine discharge.
Front. Mar. Sci. 7, 573.
https://doi.org/10.3389/fmars.2020.00573
Jain, V., Shankar, D., Vinayachandran, P. N., et al. 2017.
Evidence for the
existence of Persian Gulf water and Red Sea water in the Bay of Bengal.
Clim. Dynam. 48, 3207–3226.
https://doi.org/10.1007/s00382-016-3259-4
John, V.C., Coles, S.L., Abozed, A.I., 1990.
Seasonal cycles of
temperature, salinity and water masses of the western Arabian Gulf.
Oceanologica Acta 13 (3), 273–281.
Johns, W.E, Jacob, G.A., Kindle, J.C., Murray, S.B., Carron, M., 1999.
Arabian marginal seas and gulfs. Workshop Rep., Stennic Space Centre,
University of Miami RSMAS Technical Report 2000-01, 11–13 May, Mississippi.
Johns, W.E., Yao, F., Olson, D.B., Josey, S.A., Grist, J.P., Smeed, D.A., 2003.
Observations of seasonal exchange through the Straits of Hormuz and the
inferred heat and freshwater budgets of the Persian Gulf. J. Geophys. Res.
108(C12).
Joydas, T.V., Krishnakumar, P.K., Qurban, M.A., Ali S.M., Al- Suwailem, A.,
Al-Abdulkader, K., 2011.
Status of macrobenthic community of
Manifa–Tanajib Bay System of Saudi Arabia based on a once-off sampling
event. Mar. Pollut. Bull. 62, 1249–1260.
Joydas, T.V., Qurban M.A., Borja, A., Manokaran, S., Manikandan, K.P., Lotfi,
J.R., Garmendia J.M., Asharaf T.T.M., Ayranci, K., Shemsi, A.M, Mohammed, S.,
Basali, A.U., Panickan, P., Nazeer, Z., Lyla, P.S., Khan, S.A., Krishnakumar,
P.K., 2023.
Ecological status of macrobenthic communities in the Saudi
waters of the western Arabian Gulf. Reg. Stud. Mar. Sci. 57, 102751,
2352–4855.
https://doi.org/10.1016/j.rsma.2022.102751
Joydas, T.V., Qurban, M.A., Manikandan, K.P., et al. 2015.
Status of
macrobenthic communities in the hypersaline waters of the Gulf of Salwa,
Arabian Gulf. J. Sea Res. 99, 34–46.
https://doi.org/10.1016/j.seares.2015.01.006
Kämpf, J., Sadrinasab, M., 2006.
The circulation of the Persian Gulf: a
numerical study. Ocean Sci. 2 (1), 27–41.
https://doi.org/10.5194/os-2-27-2006
KFUPM/RI., 1990a.
Final Report: Aramco Sustaining Research Project –
Environmental Studies, Vol. VI, Oceanographic Investigation – Coastal
and Offshore Hydrography. Prepared for Saudi Aramco by the Water Resources and
Environment Division, Research Institute, King Fahd University Petroleum and
Minerals, Dhahran, Saudi Arabia, Report Project No. 24079.
KFUPM/RI., 1990b. Final Report: Aramco Sustaining Research Project –
Environmental Studies, Vol. VII,
Hydrodynamic Models for Wind-Driven and
Tidal Circulation in the Arabian Gulf. Prepared for Saudi Aramco by the
Water Resources and Environment Division, Research Institute, King Fahd
University Petroleum and Minerals, Dhahran, Saudi Arabia, Report Project No.
24079.
KFUPM/RI., 1990c. Final Report: Aramco Sustaining Research Project –
Environmental Studies, Vol. VIII,
Simulation Models of Pollutant Fate and
Transport in the Arabian Gulf. Prepared for Saudi Aramco by the Water
Resources and Environment Division, Research Institute, King Fahd University
Petroleum and Minerals, Dhahran, Saudi Arabia, Report Project No. 24079.
Le Provost, C., 1983.
Models for tides in the KAP region. [In:]
Oceanographic Modeling of the Kuwait Action Plan Region, M.I. El-Sabh (Ed),
Vol. 28, UNESCO Rep. Mar. Sci., Paris, 37–45.
L’Hégaret, P., Marez, C.D., Morvan, M., Meunier, T., Carton, X., 2021.
Spreading and vertical structure of the Persian Gulf and Red Sea outflows
in the northwestern Indian Ocean. J. Geophys. Res. 126 (4), e2019JC015983.
https://doi.org/10.1029/2019JC015983
Madah, F., Gharbi, S.H., 2022.
Numerical simulation of tidal hydrodynamics
in the Arabian Gulf. Oceanologia 64 (2), 327–345.
Marin, M., Bindoff, N.L., Feng, M., Phillips, H.E., 2021.
Slower long-term
coastal warming drives dampened trends in coastal marine heatwave
exposure. J. Geophys. Res. 126, e2021JC017930.
https://doi.org/10.1029/2021JC017930
Mussa, A.A., Aboobacker, V.M., Abdulla, C.P., Hasna, V.M., Al-Ansari, E.M.,
Vethamony, P., 2024.
A climatological overview of surface currents in the
Arabian Gulf with special reference to the Exclusive Economic Zone of
Qatar. Int. J. Climatol. 44 (13), 4677–4693.
Prasad, T.G., Ikeda, M., Kumar, S.P., 2001.
Seasonal spreading of the
Persian Gulf Water mass in the Arabian Sea. J. Geophys. Res. 106 (C8),
17059–17071.
https://doi.org/10.1029/2000JC000480
Pokavanich, T., Polikarpov, I., Lennox, A., et al. 2013.
Comprehensive
investigation of summer hydrodynamic and water quality characteristics of
desertic shallow water body: Kuwait Bay. J. Coast. Dynam. 12 (2),
1253–1264.
Pous, S., Carton, X., Lazure, P., 2013.
A process study of the wind-induced
circulation in the Persian Gulf. Open J. Mar. Sci. 3 (1), 1–11.
Rakib, F., Al-Ansari, E.M., Husrevoglu, Y.S., Yigiterhan, O., Al- Maslamani,
I., Aboobacker, V.M., Vethamony, P., 2021.
Observed variability in physical
and biogeochemical parameters in the central Arabian Gulf. Oceanologia 63
(2), 227–237.
https://doi.org/10.1016/j.oceano.2020.12.003
Reynolds, R.M., 1993.
Physical oceanography of the Gulf, Strait of Hormuz
and the Gulf of Oman – Results from the Mt. Mitchell Expedition. Mar.
Pollut. Bull. 27, 35–37.
Roy, P., Rao, I.N., Martha, T.R., Kumar, K.V., 2022.
Discharge water
temperature assessment of thermal power plant using remote sensing
techniques. Energy Geosci. 3 (2), 172–181.
https://doi.org/10.1016/j.engeos.2021.06.006
Schlitzer, R., 2003.
Ocean Data View (ODV) mp-version 1.4
(odvmp_1.4_w32.zip).
Sheppard, C.R.C., 1993.
Physical environment of the Gulf relevant of marine
pollution: An over view. Mar. Pollut. Bull. 27, 3–8.
Siddig, N.A., Al-Subhi, A.M., Alsaafani, M.A., 2019.
Tide and mean sea
level trend in the west coast of the Arabian Gulf from tide gauges and
multi-missions satellite altimeter. Oceanologia 61 (4), 401–411.
Swift, S.A., Bower, A.S., 2003.
Formation and circulation of dense water in
the Persian/Arabian Gulf. J. Geophys. Res. 108 (1), 3004.
Thoppil, P.G., Hogan, P.J., 2010.
A modeling study of circulation and
eddies in the Persian Gulf. J. Phys. Oceanogr. 40 (9), 2122–2134.
Veerasingam, S., Al-Khayat, J.A., Aboobacker, V.M., Hamza, S., Vethamony,
P., 2020a. Sources, spatial distribution and characteristics of marine
litter along the west coast of Qatar. Mar. Pollut. Bull. 159, 111478. https://doi.org/10.1016/j.marpolbul.2020.111478
Veerasingam, S., Al-Khayat, J.A., Haseeba, K.P., Aboobacker, V.M., Hamza, S.,
Vethamony, P., 2020b. Spatial distribution, structural characterization and
weathering of tarmats along the west coast of Qatar. Mar. Pollut. Bull.
159, 111486.
Veerasingam, S., Ranjani, M., Venkatachalapathy, R., Bagaev, A., Mukhanov, V.,
Litvinyuk, D., Mugilarasan, M., Gurumoorthi, K., Guganathan, L., Aboobacker,
V.M., Vethamony, P., 2021a. Contributions of Fourier transform infrared
spectroscopy in microplastic pollution research: A review. Crit. Rev.
Environ. Sci. Tech. 51 (22), 2681–2743. https://doi.org/10.1080/10643389.2020.1807450
Veerasingam, S., Vethamony, P., Aboobacker, V.M. Giraldes, A.E., Dib, S.,
Al-Khayat, J.A., 2021b. Factors influencing the vertical distribution of
microplastics in the beach sediments around the Ras Rakan Island, Qatar.
Environ. Sci. Pollut. Res. 28, 34259–34268. https://doi.org/10.1007/s11356-020-12100-4
Yao, F., Johns, W.E., 2010. A HYCOM modeling study of the Persian Gulf: 1.
Model configurations and surface circulation. J. Geophys. Res. 115,
C11017. https://doi.org/10.1029/2009JC005781
Yoshida, J., Matsuyama, M., Senjyu, T., et al. 1998. Hydrography in the RSA
during the RT/V Umitaka-Maru cruises. [In:] Offshore Environment of the ROPME
Sea Area after the War-Related Oil Spill – Results of the 1993–94
Umitaka-Maru Cruise, Otsuki, A., Abdeulraheem, M., Reyolds, M. (Eds.),
Terra Sci. Publ. Company, Tokyo, 1–22.
Statistical downscaling of global climate projections over the Egyptian Red Sea coast
Oceanologia, 67 (4)/2025, 67408, 21 pp.
https://doi.org/10.5697/DZVJ9279
Mohamed Shaltout1, Ahmed Abdelhamid1, Ahmed Adel1, Mohamed Gad1,2, Mohamed Elbessa*,1,3
1Oceanography Department, Faculty of Science, Alexandria University, Alexandria, Egypt;
e-mail: Mohamed.Abdelhameed_PG@alexu.edu.eg (M. Elbessa)
2National Institute of Oceanography and Fisheries, NIOF, Cairo, Egypt
3College of Maritime Transport and Technology (CMTT), Arab Academy for Science, Technology and Maritime Transport (AASTMT), Abu-Qir, Alexandria, Egypt
*corresponding author
Keywords:
Red Sea; Statistical downscaling; Ensemble mean; SSPs; Climate conditions
Received: 17 September 2024; revised: 17 September 2025; accepted: 25 September 2025
Highlights
- Statistical Downscaling provides powerful scientific tools to study the climatic parameters (surface air temperature, surface relative humidity, surface wind regime, and mean sea level pressure) along the Egyptian Mediterranean coast.
- This study described in detail the short and long-period atmospheric characteristics.
- The study area showed marked monthly and spatial variabilities.
- The current research opens a scientific vision to deal with the current climate data for any future mitigation and adaptation scenarios.
Abstract
The long-term variability in atmospheric parameters plays a crucial role in shaping regional climate change. This study
investigates the current and future characteristics of different surface atmospheric properties over the period 2011–2100
along the Egyptian Red Sea coast. First, the observed data were used to describe the short-term variability of weather
conditions (2011–2021). Second, a bias correction statistical model based on the cumulative distribution functions
(CDF) technique was developed by matching the four climate models [GFDL-ESM4; IPSL-CM6A-LR; MIROC6; MRI-ESM2-0] individually with daily observations over a 12-year overlapping period. Third, the resulting bias-correction models were applied to statistically downscale future projections of the studied atmospheric parameters under the Shared Socioeconomic Pathways (SSPs) scenarios. After bias correction, the outputs of each used climate model were averaged to calculate the ensemble mean for the period 2015–2100, improving accuracy and validity along ERSC at each of the five studied stations.
Our results indicate that, following bias correction the future scenarios of the Shared Socioeconomic Pathways (SSPs)
show that the Egyptian Red Sea coast will experience significant changes with a wide range of uncertainty, with trends
ranging from −0.14 to 2.91°C century−1, −0.021 to 0.069 m s−1 century−1, and −1.95 to 0.67 hPa century−1 for surface air temperature, wind speed, and sea level pressure, respectively.
Graphical Abstract
Statistical Downscaling of Global Climate Projections Over the Egyptian
Red Sea Coast
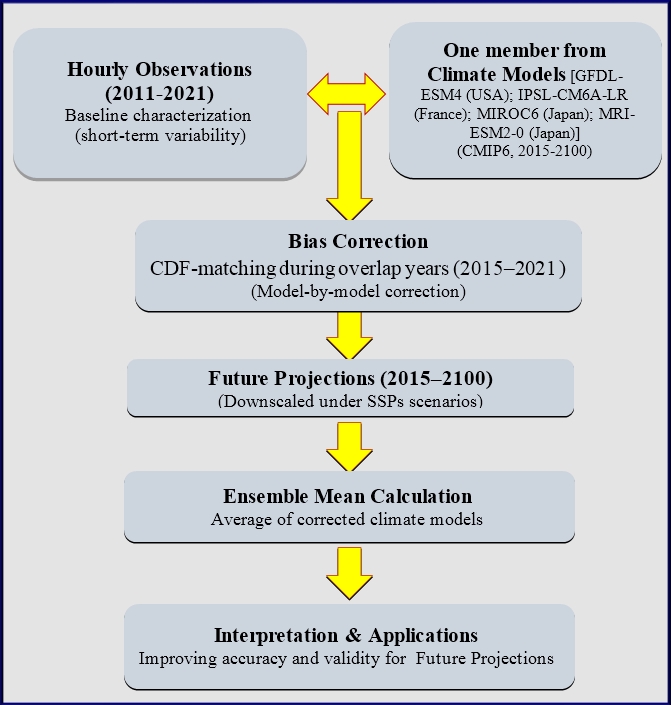
References 
Abualnaja, Y., Papadopoulos, V.P., Josey, S.A., Hoteit, I., Kontoyiannis, H.,
Raitsos, D.E., 2015.
Impacts of climate modes on air–sea heat exchange in
the Red Sea. J. Climate 28 (7), 2665–2681.
https://doi.org/10.1175/JCLI-D-14-00379.1
Acker, F., 2008.
New findings on unconscious versus conscious thought in
decision making: additional empirical data and meta-analysis. Judgment.
Decis. Make. 3 (4), 292–303.
https://doi.org/10.1017/S1930297500000863
Al-Barakati, A.M., James, A.E., Karakas, G., 2002.
A threedimensional
hydrodynamic model to predict the distribution of temperature, salinity, and
water circulation of the Red Sea. J. King Abdulaziz Univ. Mar. Sci. 13
(1). 3–16.
https://doi.org/10.4197/MAR.13-1.1
Al-Subhi, A.M., Al-Aqsum, M.M., 2008.
Temporal and spatial variations of
remotely sensed Sea surface temperature in the northern Red Sea. J. King
Abdulaziz Univ. Mar. Sci. 19 (1), 61–74.
https://doi.org/10.4197/mar.19-1.5
Ayman, M., Salah, Z., Tonbol, K., Shaltout, M., 2023.
Evaluating ERA5
Weather Parameters Data Using Remote Sensing and in Situ Data Over the North
Red Sea. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 48, 77–84.
https://doi.org/10.5194/isprs-archives-XLVIII-1-W2-2023-77-2023
Bawadekji, A., Tonbol, K., Ghazouani, N., Becheikh, N., Shaltout, M., 2022.
Recent atmospheric changes and future projections along the Saudi Arabian
Red Sea Coast. Sci. Rep.12 (1), 160.
https://doi.org/10.1038/s41598-021-04200-z
Boucher, O., Denvil, S., Levavasseur, G., Cozic, A., Caubel, A., Foujols, M.A.,
Meurdesoif, Y., Gastineau, G., 2018.
IPSL IPSL-CM6A-LR model output
prepared for CMIP6 CMIP. Earth System Grid Federation.
https://doi.org/10.22033/esgf/cmip6.1534
Bruckner, A., Rowlands, G., Riegl, B., Purkis, S., Williams, A., Renaud, P.,
2012. Atlas of Saudi Arabian Red Sea Marine Habitats. Panoramic Press Phoenix.
AZ, USA. Chaidez, V., Dreano, D., Agusti, S., Duarte, C.M., Hoteit, I., 2017.
Decadal trends in Red Sea maximum surface temperature. Sci. Rep.
7:8144, 1–8.
https://doi.org/10.1038/s41598-017-08146-z
Dawod, G., Amin, A., Haggag, G.G., 2022.
Variations of sea levels and
atmospheric parameters along the Egyptian coasts over 2008–2020. J. Sci.
Eng. Res. 9 (5), 85–100.
Edwards, F.J., 1987.
Climate and Oceanography, Key Environments: Red
Sea. Pergamon Press, Oxford. 1, 45–68.
El Saman, M.I., Mahmoud, M.A., 2016.
Factors Affecting the Weather and
Oceanography Parameters in Different Structural Areas of the Red Sea,
Egypt. Univ. J. Environ. Res. Technol. 6(2), 43–53.
Eladawy, A., Nadaoka, K., Negm, A., Abdel-Fattah, S., Hanafy, M., Shaltout, M.,
2017.
Characterization of the northern Red Sea’s oceanic features with
remote sensing data and outputs from a global circulation model.
Oceanologia 59 (3), 213-237. https://doi.org/10.1016/j.oceano.2017.01.002
ElBessa, M., Abdelrahman, S.M., Tonbol, K., Shaltout, M., 2021.
Dynamical
downscaling of surface air temperature and wind field variabilities over the
Southeastern Levantine basin, Mediterranean Sea. Climate 9 (10), 150.
https://doi.org/10.3390/cli9100150
ElBessa, M., Shaltout, M., 2024.
Statistical downscaling of global climate
projections along the Egyptian Mediterranean coast. Oceanologia 66 (4),
66401, 25 pp.
https://doi.org/10.5697/OBOE5006
Fowler, H.J., Blenkinsop, S., Tebaldi, C., 2007.
Linking climate change
modeling to impacts studies: Recent advances in downscaling techniques for
hydrological modeling. Int. J. Climatol. 27(12), 1547–1578.
https://doi.org/10.1002/joc.1556
Fouda, M.M., Gerges, M.A., 1994.
Implications of climate change in the Red
Sea and Gulf of Aden region: an overview.
Gad, M., Eid, F., El-Din, S.S., Radwan, A., Soliman, G., 2019.
Estimation
of the Salt Storage and the Salt Content in the Gulf of Suez. J. King
Abdulaziz Univ. Mar. Sci. 29 (1), 37–51.
https://doi.org/10.4197/Mar.29-1.3
Hamed, M.M., Salehie, O., Nashwan, M.S., Shahid, S., 2023.
Projection of
temperature extremes of Egypt using CMIP6 GCMs under multiple shared
socioeconomic pathways. Environ. Sci. Pollut. Res.30, 38063–38075.
https://doi.org/10.1007/s11356-022-24985-4
Hochman, A., Marra, F., Messori, G., Pinto, J. G., Raveh-Rubin, S., Yosef, Y.,
Zittis, G., 2022.
Extreme weather and societal impacts in the eastern
Mediterranean. Earth Syst. Dynam. 13 (2), 749–777.
https://doi.org/10.5194/esd-13-749-2022
Jiang, H., Farrar, J.T., Beardsley, R.C., Chen, R., Chen, C., 2009.
Zonal
surface wind jets across the Red Sea due to mountain gap forcing along both
sides of the Red Sea. Geophys. Res. Lett. 36, L19605.
https://doi.org/10.1029/2009GL040008
Kaunang, T., Medellu, C.S., 2013.
Fluctuation of daytime air humidity in
the mangrove forest edges. J. Biol. Agric. Healthc. 3 (13), 154–159.
Krasting, J.P., John, J.G., Blanton, C., McHugh, C., Nikonov, S.,
Radhakrishnan, A., Rand, K., Zadeh, N.T., Balaji, V., Durachta, J., Dupuis, C.,
2018.
NOAA-GFDL GFDL-ESM4 model output prepared for CMIP6 CMIP. Earth
System Grid Federation.
https://doi.org/10.22033/ESGF/CMIP6.1407
Langodan, S., Cavaleri, L., Vishwanadhapalli, Y., Pomaro, A., Bertotti, L.,
Hoteit, I., 2017.
IPCC, 2023: The climatology of the Red Sea–part 1: the
wind. Int. J. Climatol. 37, 4509–4517.
https://doi.org/10.1002/joc.5103
Lee, H., Calvin, K., Dasgupta, D., Krinner, G., Mukherji, A., Thorne, P.W.,
Trisos, C., Romero, J., Aldunce, P., Barrett, K., Blanco, G., Cheung, W.W.,
2023.
Climate change 2023: synthesis report. IPCC, 2023: Contribution of
working groups I, II, and III to the sixth assessment report of the
Intergovernmental Panel on Climate Change. The Australian Nat. Univ.
Maraun, D., Wetterhall, F., Ireson, A.M., Chandler, R.E., Kendon, E.J.,
Widmann, M., Brienen, S., Rust, H.W., Sauter, T., Themeßl, M., Venema, V.K.C.,
2010.
Precipitation downscaling under climate change: Recent developments
to bridge the gap between dynamical models and the end user. Rev. Geophys.
48(3). RG3003.
https://doi.org/10.1029/2009RG000314
Menezes, V.V., Farrar, J.T., Bower, A.S., 2018.
Westward mountain-gap wind
jets of the northern Red Sea as seen by QuikSCAT. Remote Sens. Environ.
209, 677–699.
https://doi.org/10.1016/j.rse.2018.02.075
Michel, D., Pandya, A., 2010.
Coastal zones and climate change Henry
L. Stimson Center, Washington, 106 pp.
Middleton, N.J., Thomas, D.S., 1992.
World atlas of desertification.
Mohammed, T., Dar, M.A., El-Saman, M.I., 2010.
Distribution patterns of hard and soft corals along the Egyptian Red Sea Coast. Egypt. J. Aquat. Res. 36, 543–555.
Patzert, W.C., 1974.
Wind-induced reversal in Red Sea circulation.
Deep-Sea Res. Oceanogr. Abstr. 21 (2), 109–121.
https://doi.org/10.1016/0011-7471(74)90068-0
Pedgley, D., 1974.
An outline of the weather and climate of the Red
Sea. Océanogr. Phys. Mer Rouge Paris, France, UNESCO, 9–27.
Sherwood, S.C., Huber, M., 2010.
An adaptability limit to climate change
due to heat stress. Proc. Natl. Acad. Sci. 107 (21), 9552–9555.
https://doi.org/10.1073/pnas.0913352107
Sofianos, S.S., Johns, W.E., 2007. Observations of the summer Red Sea
circulation. J. Geophys. Res. 112, C06025. https://doi.org/10.1029/2006JC003886
Tatebe, H., Watanabe, M., 2018. MIROC MIROC6 model output prepared for
CMIP6 CMIP. Earth System Grid Federation. https://doi.org/10.22033/ESGF/CMIP6.881
Timbal, B., Fernandez, E., Li, Z., 2009. Generalization of a statistical
downscaling model to provide local climate change projections for Australia,
Environmental Modelling and Software. Environ. Model. Softw. 24 (3),
341–358. https://doi.org/10.1016/j.envsoft.2008.07.007
Tonbol, K.M., El-Geziry, T.M., Elbessa, M., 2019. Assessment of weather
variability over Safaga harbor, Egypt. Arab. J. Geosci. 12, 805. https://doi.org/10.1007/s12517-019-4974-z
Tonbol, K., 2024. Climate change: interdisciplinary solutions for a global
challenge. Multidisc. Adaptive Clim. Insights 1(1), 1–10. https://doi.org/10.21622/MACI.2024.01.1.907
Turki, J.A., Al-Subh, A.M., Madah, F., 2023. Influence of Tokar Gap wind jet on
latent heat flux of Central Red Sea: empirical orthogonal function approach.
Ocean Coast. Res. 71(13), e23029. https://doi.org/10.1590/2675-2824071.220103jat
United Nations Educational, Scientific, and Cultural Organization, 1979.
Map of the world distribution of arid regions. MAB Tech. Notes 7,
UNESCO, Paris, 54 pp.
Vaittinada, A.P., Vrac, M., Mailhot, A., 2021. Ensemble bias correction of
climate simulations: preserving internal variability. Sci Rep. 11(1),
3098. https://doi.org/10.1038/s41598-02182715-1
Vigaud, N., Varc, M., Caballero, Y., 2013. Probabilistic downscaling of GCM
senarios over southern India. Int. J. Climatol. 33 (5), 1248–1263. https://doi.org/10.1002/joc.3509
Viswanadhapalli, Y., Dasari, H.P., Langodan, S., Challa, V.S., Hoteit, I.,
2017. Climatic features of the Red Sea from a regional assimilative
model. Int. J. Climat. 37, 2563–2581. https://doi.org/10.1002/joc.4865
Wilby, R.L., Wigley, T.M.L., 1997. Downscaling general circulation model
output: A review of methods and limitations. Prog. Phys. Geogr. 21(4),
530–548. https://doi.org/10.1177/030913339702100403
Yukimoto, S., Koshiro, T., Kawai, H., Oshima, N., Yoshida, K., Urakawa, S.,
Tsujino, H., Deushi, M., Tanaka, T., Hosaka, M., Yoshimura, H., 2019.
MRI-ESM2.0 model output prepared for CMIP6 CMIP. Earth System Grid
Federation. https://doi.org/10.22033/ESGF/CMIP6.621
Benthic diatom communities in deeper areas of the German Baltic Sea
Oceanologia, 67 (4)/2025, 67409, 13 pp.
https://doi.org/10.5697/CRPJ6914
Marjan Janßen1, Israel Barrantes2, Mirko Dressler3,4, Karin Glaser5, Ulf Karsten1,6,*
1Institute for Biological Sciences, Applied Ecology and Phycology, University of Rostock, Albert-Einstein-Strasse 3, D–18051 Rostock, Germany;
e-mail: ulf.karsten@uni-rostock.de (U. Karsten)
2Institute for Biostatistic and Informatics in Medicine and Ageing Research, University of Rostock, Schillingallee 35, D–18057 Rostock, Germany
3Physical Geography, Institute for Geography and Geology, University of Greifswald, Friedrich-Ludwig-Jahn Str. 16, 17487 Greifswald, Germany
4Institute for Biological Sciences, Department of Botany, University of Rostock, Wismarsche Straße 44/44, D–18051 Rostock, Germany
5Karin Glaser, Faculty for Chemistry, Physics and Biosciences, Institute for Biosciences, Department of Biology/Ecology, TU Bergakademie Freiberg, D–09599 Freiberg, Germany
6Interdisciplinary Faculty, Department of Maritime Systems, University of Rostock, D–18051, Rostock, Germany
*corresponding author
Keywords:
Microphytobenthos; Diatoms; Baltic Sea
Received: 17 April 2025; revised: 26 September 2025; accepted: 2 October 2025
Highlights
- This study represents the first investigation of the biodiversity of microphytobenthic communities in deeper offshore regions of the Southern Baltic Sea.
- At these stations, the number and abundance of benthic species decreased with increasing depth.
- The microphytobenthic communities differed in species composition across the study sites, with numerous taxa that could not be identified.
- Even at depths of 38 m, vital benthic diatoms could be observed. This phenomenon might be explained by their low light requirements for photosynthesis in combination with high physiological plasticity.
Abstract
The Baltic Sea is a shallow, semi-enclosed brackish ecosystem in northern Europe, which is strongly affected by climate
change and other anthropogenic disturbances such as mobile bottom trawling. The resulting drag forces exerted by such fishing practice physically disturb the sea bed and impact all benthic organisms such as microphytobenthic communities, which represent key primary producers in marine soft-bottom ecosystems. Despite their ecological importance, little is known about the composition and productivity of these benthic communities in deeper areas of the German Baltic Sea. Therefore, this study investigates the occurrence and diversity of benthic diatoms in such unstudied areas, focusing on the Baltic Sea regions Fehmarnbelt, Rönnebank, and Oderbank. Sediment cores were collected from depths down to 36 meters, processed ex-situ and the biodiversity of benthic diatoms evaluated using morphological traits via light microscopy and high-throughput sequencing. The data provide novel insights on the occurrence of benthic diatom communities in deeper areas of the Baltic Sea and these microalgae seem to be suitable bioindicators to document any sediment disturbance by natural or anthropogenic forces.
References 
Antonelli, M., Wetzel, C. E., Ector, L., Teuling, A. J., Pfister, L., 2017.
On the potential for terrestrial diatom communities and diatom indices to
identify anthropic disturbance in soils. Ecol. Indic. 75, 73–81.
https://doi.org/10.1016/j.ecolind.2016.12.003
Ask, J., Rowe, O., Brugel, S., Strömgren, M., Byström, P., Andersson, A.,
2016
. Importance of coastal primary production in the northern Baltic
Sea. Ambio 45, 635–648.
https://doi.org/10.1007/s13280-016-0778-5
Barry, R. G., Hartigan, P. J., 1999.
Vegan: Community Ecology Package.
R Package Version 2.5-6.
https://CRAN.R-project.org/package=vegan
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al
Ghalith, G. A., Caporaso, J. G., 2019.
Reproducible, interactive, scalable
and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37,
852–857.
https://doi.org/10.1038/s41587-019-0209-9
Bradley, I.M., Pinto, A.J., Guest, J.S., 2016.
Design and evaluation of
Illumina MiSeq-compatible, 18S rRNA genespecific primers for improved
characterization of mixed phototrophic communities. Appl. Environ.
Microbiol. 82.
https://doi.org/10.1128/AEM.01630-16
Brown, M. R., GrDunstan, G. A., Norwood, S. J., Miller, K. A., 1969.
Effects of harvest stage and light on the biochemical composition of the
Diatom Thalassiosira pseudonana. J. Phycol. 32, 64–73.
https://doi.org/10.1111/j.0022-3646.1996.00064.x
Cahoon, L. B., 1999.
The role of benthic microalgae in neritic
ecosystems. Oceanogr. Mar. Biol. 37, 40–86.
https://doi.org/10.1201/9781482298550-4
Callahan, B.J., McMurdie, P.J., Han, A.W., Johnson, A.J., Holmes, S.P., 2016.
DADA2: High-resolution sample inference from Illumina amplicon data.
Nat. Methods 13, 581–583.
https://doi.org/10.1038/nmeth.3869
Christianen, M. J., Middelburg, J. J., Holthuijsen, S. J., Jouta, J., Compton,
T. J., van der Heide, T., Schouten, S., Olff, H., 2017.
Benthic primary
producers are key to sustain the Wadden Sea food web: stable carbon isotope
analysis at landscape scale. Ecology 98, 1498–1512.
https://doi.org/10.1002/ecy.1837
Collie, J.S., Hall, S.J., Kaiser, M.J., Poiner, I.R., 2000.
A quantitative
analysis of fishing impacts on shelf-sea benthos. J. Anim. Ecol. 69,
785–798.
https://doi.org/10.1046/j.1365-2656.2000.00434.xx
de Groot, S., 1984.
The impact of bottom trawling on benthic fauna of the
North Sea. Ocean Manag. 9, 177–190.
https://doi.org/10.1016/0302-184X(84)90002-7
Glud, R. N., Woelfel, J., Karsten, U., Kühl, M., Rysgaard, S., 2009.
Benthic microalgal production in the Arctic: Applied methods and status of
the current database. Bot. Mar. 52, 559–571.
https://doi:10.1515/BOT.2009.074
Guiry, M.D., Guiry, G.M., 2025.
AlgaeBase. World-wide electronic
publication, University of Galway.
https://www.algaebase.org
Hendey, N.I., 1965.
An Introductory Account of the Smaller Algae of British
Coastal Waters. Part V. Bacillariophyceae (Diatoms). J. Mar. Biol. Assoc.
U.K. 45, 798.
https://doi.org/10.1017/S0025315400016660
Hope, J. A., Paterson, D. M., Thrush, S. F., 2019.
The role of
microphytobenthos in soft-sediment ecological networks and their contribution
to the delivery of multiple ecosystem services. J. Ecol. 108 (3).
https://doi.org/10.1111/1365-2745.13322
Hoppe, C.J.M., Fuchs, N., Notz, D. et al., 2024.
Photosynthetic light
requirement near the theoretical minimum detected in Arctic microalgae.
Nat. Commun. 15, 7385.
https://doi.org/10.1038/s41467-024-51636-8
Karsten, U., Kuriyama, K., Hübener, T., Woelfel, J., 2021.
Benthic
Diatoms on Sheltered Coastal Soft Bottoms (Baltic Sea) – Seasonal Community
Production and Respiration. J. Mar. Sci. Eng. 9, 949.
https://doi.org/10.3390/jmse9090949
Kelly, M., Bennion, H., Burgess, A., Ellis, J., Juggins, S., Guthrie, R.,
Yallop, M., 2009.
Uncertainty in ecological status assessments of lakes and
rivers using diatoms. Hydrobiologia, 627, 5–15.
https://doi.org/10.1007/s10750-009-9872-z
Kirk, J.T.O., 1994.
Light and Photosynthesis in Aquatic Ecosystems.
Cambridge University Press, Cambridge, 509 pp.
https://doi.org/10.1017/CBO9780511623370
Kuriyama, K., Gründling-Pfaff, S., Diehl, N., Woelfel, J., Karsten, U., 2021.
Microphytobenthic primary production on exposed coastal sandy sediments of
the Southern Baltic Sea using ex situ sediment cores and oxygen optodes.
Oceanologia 63, 247–260.
https://doi.org/10.1016/j.oceano.2021.02.002
Kuriyama, K., Heesch, S., Karsten, U., Schumann, R., 2023.
Benthic diatom
diversity in a turbid brackish lagoon of the Baltic Sea. Phycologia, 62,
164–178.
https://doi.org/10.1080/00318884.2022.2151288
Kwandrans, J., Eloranta, P., Kawecka, B., Wojtan, K., 1998.
Use of benthic
diatom communities to evaluate water quality in rivers of southern Poland.
J. Appl. Phycol. 10, 193–201.
https://doi.org/10.1023/A:1008087114256
Lindström, M., 2000.
Seasonal Changes in the Underwater Light Milieu in a
Finnish Baltic. Geophysica 236, 15–232.
Luhtala, H., Tolvanen, H., Kalliola, R., 2013.
Annual spatiotemporal
variation of the euphotic depth in the SWFinnish archipelago, Baltic Sea.
Oceanologia 55, 359–373.
https://doi.org/10.5697/oc.55-2.359
Lundkvist, M., Grue, M., Friend, P. L., Flindt, M. R., 2007.
The relative
contributions of physical and microbiological factors to cohesive sediment
stability. Cont. Shelf Res. 27, 1143–1152.
https://doi.org/10.1016/j.csr.2006.01.021
McGee, D., Laws, R.A., Cahoon, L.B., 2008.
Live benthic diatoms from the
upper continental slope: extending the limits of marine primary
production. Mar. Ecol. Prog. Ser. 356, 103–112.
https://doi.org/10.3354/meps07280
McMurdie, P.J., Holmes, S., 2013.
phyloseq: an R package for reproducible
interactive analysis and graphics of microbiome census data. PLoS One 8
(4), e61217.
https://doi.org/10.1371/journal.pone.0061217
Miller, D.C., Geider, R.J., MacIntyre, H.L., 1996.
Microphytobenthos: The
ecological role of the “secret garden” of unvegetated, shallow-water marine
habitats. II. role in sediment stability and shallow-water food webs.
Estuaries Coast. 19, 202–212.
https://doi.org/10.2307/1352224
Nelson, D.M., Tréguer, P., Brzezinski, M.A., Leynaert, A., Quéguiner, B.,
1995
. Production and dissolution of biogenic silica in the ocean: Revised
global estimates, comparison with regional data and relationship to biogenic
sedimentation. Global Biogeochem. Cy. 9, 359–372.
https://doi.org/10.1029/95GB01070
Oberle, F.K., Storlazzi, C.D., Hanebuth, T.J., 2016.
What a drag:
Quantifying the global impact of chronic bottom trawling on continental shelf
sediment. J. Mar. Syst. 159, 109–119.
https://doi.org/10.1016/j.jmarsys.2015.12.007
O’Neill, F., Summerbell, K.D., 2016.
The hydrodynamic drag and the
mobilisation of sediment into the water column of towed fishing gear
components. J. Mar. Syst. 164, 76–84.
https://doi.org/10.1016/j.jmarsys.2016.08.008
Palanques, A., Guillén, J., Puig, P., 2001.
Impact of bottom trawling on
water turbidity and muddy sediment of an unfished continental shelf.
Limnol. Oceanogr. 46, 1100–1110.
https://doi.org/10.4319/lo.2001.46.5.1100
Paterson, D.M., 1989.
Short-term changes in the erodibility of intertidal
cohesive sediments related to the migratory behavior of epipelic diatoms.
Limnol. Oceanogr. 34, 223–234.
https://doi.org/10.4319/lo.1989.34.1.0223
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V., Thirion, B., Grisel,
O., Cournapeau, D., 2011.
Scikit-learn: Machine Learning in Python. J.
Mach. Learn. Res. 12, 2825–2830.
https://doi.org/10.48550/arXiv.1201.0490
Prelle, L.R., Karsten, U., 2022.
Photosynthesis, Respiration, and Growth of
Five Benthic Diatom Strains as a Function of Intermixing Processes of Coastal
Peatlands with the Baltic Sea. Microorganisms, 10, 749.
https://doi.org/10.3390/microorganisms10040749
Prelle, L.R., Albrecht, M., Karsten, U., Damer, P., Giese, T., Jähns, J.,
Glaser, K., 2021.
Ecophysiological and Cell Biological Traits of Benthic
Diatoms from Coastal Wetlands of the Southern Baltic Sea. Front.
Microbiol. 12, 642–811.
https://doi.org/10.3389/fmicb.2021.642811
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P.,
Glöckner, F. O., 2013.
The SILVA ribosomal RNA gene database project:
improved data processing and web-based tools. Nucleic Acids Res. 41,
590–596.
https://doi.org/10.1093/nar/gks1219
Ragueneau, O., Schultes, S., Bidle, K., Claquin, P., Moriceau, B., 2006.
Si
and C interactions in the world ocean: Importance of ecological processes and
implications for the role of diatoms in the biological pump. Global
Biogeochem. Cy. 20, GB4S02.
https://doi.org/10.1029/2006GB002688
Raven, J. A., Kübler, J. E., Beardall, J., 2000.
Put out the light, and
then put out the light. J. Mar. Biol. Assoc. UK. 80, 1–25.
https://doi:10.1017/S0025315499001526
Robinson, D.H., Arrigo, K.R., Iturriaga, R., Sullivan, C.W., 1995.
Microalgal light-harvesting in extreme low-light environments in McMurdo
Sound, Antarctica. J. Phycol. 31, 508–520.
https://doi.org/10.1111/j.1529-8817.1995.tb02544.x
Salonen, A., Salojärvi, J., Lahti, L., de Vos, W., 2012.
The adult
intestinal core microbiota is determined by analysis depth and health
status. Clin. Microbiol. Infect. 18, 16–20.
https://doi.org/10.1111/j.1469-0691.2012.03855.x
Savchuk, O.P., 2018.
Large-Scale Nutrient Dynamics in the Baltic Sea,
1970–2016. Front. Mar. Sci. 5, 95.
https://doi.org/10.3389/fmars.2018.00095
Schubert, H., Sagert, S., Forster, R.M., 2001.
Evaluation of the different
levels of variability in the underwater light field of a shallow estuary.
Helgoland Mar. Res. 55, 12–22.
https://doi.org/10.1007/s101520000064
Schultz, K., Dreßler, M., Karsten, U., Mutinova, P.T., Prelle, L.R., 2024.
Benthic diatom community response to the sudden rewetting of a coastal
peatland. Sci. Total Environ. 955, 177053.
https://doi.org/10.1016/j.scitotenv.2024.177053
Sciberras, M., Hiddink, J.G., Jennings, S., Szostek, C.L., Hughes, K.M.,
Kneafsey, B., Kaiser, M.J., 2018.
Response of benthic fauna to experimental
bottom fishing: A global meta-analysis. Fish. Aquat. Ecol. 19, 698–715.
https://doi.org/10.1111/faf.12283
Serôdio, J., Paterson, D.M., 2022
. Role of microphytobenthos in the
functioning of estuarine and coastal ecosystems. [In:] Life below water,
Springer International Publishing, Cham, 894–906.
Shetty, S.A., Hugenholtz, F., Lathi, L., Schmidt, H., de Vos, W.M., 2017.
Intestinal microbiome landscaping: insight in community assemblage and
implications for microbial modulation strategies. FEMS Microbiol. Rev. 41,
182–199.
https://doi.org/10.1093/femsre/fuw045
Stachura-Suchoples, K., 2001.
Bioindicative values of dominant diatom
species from the Gulf of Gdansk (Southern Baltic Sea). [In:] Studies on
Diatoms. Jahn, R., Kociolek, J.P., Witkowski, A., Compére P. (Eds).
Lange-Bertalot-Festschrift, Koeltz Scientific Books, Königstein, 477–490.
Tauber, F., Lemke, W., 1995.
Map of sediment distribution in the Western
Baltic Sea (1:100,000), Sheet “Darß”. Deutsche Hydrogr. Z. 47,
171–178.
https://doi.org/10.1007/BF02736203
Tauber, F., Lemke, W., Endler, R., 1999.
Map of sediment distribution in
the Western Baltic Sea (1:100,000), Sheet Falster – Møn. Deutsche
Hydrogr. Z. 51, 5–32.
https://doi.org/10.1007/BF02763954
Underwood, G., Kromkamp, J., 1999.
Primary production by phytoplankton and
microphytobenthos in estuaries. Adv. Ecol. Res. 29, 93–153.
https://doi.org/10.1016/S0065-2504(08)60192-0
Urban-Malinga, B., Wiktor, J., 2003.
Microphytobenthic primary production
along a non-tidal sandy beach gradient: an annual study from the Baltic
Sea. Oceanologia 45, 705–720.
Vilbaste, S., Sundbäck, K., Nilsson, C., Truu, J., 2000.
Distribution of
benthic diatoms in the littoral zone of the Gulf of Riga, the Baltic Sea.
Eur. J. Phycol. 35, 373–385.
https://doi.org/10.1080/09670260010001735981
Villanova, V., Fortunato, E.A., Singh, D., Dal Bo, D., Conte, M., Obata, T.,
… Finazzi, G., 2017.
Investigating mixotrophic metabolism in the model
diatom Phaeodactylum tricornutum. Philos. Trans. R. Soc. Lond. B Biol.
Sci. 372 (1728), 20160404.
https://doi.org/10.1098/rstb.2016.0404
Virta, L., Soininen, J., 2017.
Distribution patterns of epilithic diatoms
along climatic, spatial and physicochemical variables in the Baltic Sea.
Helgoland Mar. Res. 71, 16.
https://doi.org/10.1186/s10152-017-0496-9
Wickham, H. 2016.
ggplot2: Elegant Graphics for Data Analysis.
Springer-Verlag, New York. Accessed at
https://ggplot2.tidyverse.org
Witkowski, A., 1994.
Recent and fossil diatom flora of the Gulf of Gdansk,
Southern Baltic Sea. Bibliotheca Diatomologica 28, J. Cramer in der
Gebrüder-Borntraeger-Verlags-Buchhandlung, Berlin, Stuttgart, 313 pp.
Witkowski, A., 2000.
Diatom Flora of Marine Coasts I. Iconographia
Diatomologica 7, Koeltz Scientific Books, Königstein, 925 pp.
Woelfel, J., Schoknecht, A., Schumann, R., Karsten, U., 2014.
Growth and
primary production characteristics of three benthic diatoms from the brackish
Southern Baltic Sea in relation to varying environmental conditions.
Phycologia 53, 639–651.
https://doi.org/10.2216/14-019.1
Zimmermann, J., Jahn, R., Gemeinholzer, B., 2011.
Barcoding diatoms:
evaluation of the V4 subregion on the 18S rRNA gene, including new primers and
protocols. Org. Divers. Evol. 11, 173–192.
https://doi.org/10.1007/s13127-011-0050-6
First record of Moerisia cf. inkermanica Paltschikowa-Ostroumowa, 1925 (Hydrozoa, Moerisiidae) in the Gulf of Gdańsk (southern Baltic Sea)
Oceanologia, 67 (4)/2025, 67410, 8 pp.
https://doi.org/10.5697/IZOO2792
Michał Olenycz*, Marcin Kalarus
Gdynia Maritime University, Roberta de Plelo 20, 80-548, Gdańsk, Poland;
e-mail: molenycz@im.umg.edu.pl (M. Olenycz)
*corresponding author
Keywords:
Hydromedusae; Non-native species; Species introduction; Ballast water
Received: 17 September 2024; revised: 17 September 2025; accepted: 25 September 2025
Highlights
- First record of Moerisia cf inkermanica in the Gulf of Gdańsk (the Southern Baltic Sea).
- Possible spread mechanisms for M. cf inkermanica.
- Potential developing population of M. cf inkermanica in the southern part of the Gulf of Gdańsk.
Abstract
The hydromedusa Moerisia cf. inkermanica Paltschikowa-Ostroumowa, 1925, native to the Pontocaspian region,
was recorded for the first time in the Gulf of Gdańsk, in the waters of the Port of Gdańsk. In August 2024, 78 specimens (2–12 mm) were collected at three research stations. This observation constitutes the second Baltic record of the species and the first in the southern Baltic Sea. While ballast water discharge is the most likely vector for the introduction of the species, the ban on untreated ballast water discharge, which came into effect in 2020, suggests an emerging local population that warrants further investigation.
References 
Ahuatzin-Hernández, J.M., Ordóñez-López, U., Herrera-Rodrı́guez, M.,
Olvera-Novoa, M.A., 2024.
Occurrence of the hydromedusa Moerisia cf.
inkermanica (Hydrozoa, Moerisiidae) in the ballast water of oil tankers in the
Gulf of Mexico. J. Mar. Biol. Assoc. U. K. 104 (60), 1—9.
https://doi.org/10.1017/S002531542400050X
AquaNIS, 2025.
Information system on Aquatic Non-Indigenous and Cryptogenic
Species. https://aquanisresearch.com (accessed 12
April 2025)
Brulińska, D., Olenycz, M., Ziółkowska, M., Mudrak-Cegiołka, S.,
Wołowicz, M., 2016
. Moon jellyfish, Aurelia aurita, in the Gulf of Gdansk:
threatening predator or not? Boreal Environment Research 21, 528—540.
http://hdl.handle.net/10138/225818
GDEP, 2018a.
Neogobius melanostomus (Pallas, 1814). Species
Information Card. General Directorate for Environmental Protection, 7 pp.
https://www.gov.pl/web/gdos/neogobius-melanostomus---babka-bycza
(accessed 12 April 2025)
GDEP, 2018b.
Rhithropanopeus harrisii (Gould, 1841). Species
Information Card. General Directorate for Environmental Protection, 7 pp.
https://www.gov.pl/web/gdos/rhithropanopeus-harrisii---krabik-amerykanski
(accessed 12 April 2025)
GDEP, 2018c.
Mnemiopsis leidyi (L. Agassiz, 1865). Species Information
Card. General Directorate for Environmental Protection, 7 pp.
https://www.gov.pl/web/gdos/mnemiopsis-leidyi
(accessed 12 April 2025)
Haslob, H., Clemmesen, C., Schaber, M., Hinrichsen, H.-H., Schmidt, J.O., Voss,
R., Kraus, G., Köster, F.W., 2007.
Invading Mnemiopsis leidyi as a
potential threat to Baltic fish. Mar. Ecol. Prog. Ser. 349, 303—306.
https://doi.org/10.3354/meps07283
HELCOM, 2013.
HELCOM ALIENS 2 – Non-native species port survey protocols,
target species selection and risk assessment tools for the Baltic Sea.
Helsinki Commission, 34 pp.
HELCOM, 2014.
HELCOM Guide to Alien Species and Ballast Water Management in
the Baltic Sea, 40 pp.
Janas, U., Zgrudno, A., 2007.
First record of Mnemiopsis leidyi A. Agassiz,
1865 in the Gulf of Gdańsk (southern Baltic Sea). Aquat. Invasions 2 (4),
450—454.
https://doi.org/10.3391/ai.2007.2.4.18
Janecki, M., Dybowski, D., Jakacki, J., Nowicki, A., Dzierzbicka- Głowacka,
L., 2021.
The use of satellite data to determine the changes of
hydrodynamic parameters in the Gulf of Gdańsk via EcoFish model. Remote
Sens. 13 (18), 38 pp.
https://doi.org/10.3390/rs13183572
Jasińska, E., Robakiewicz, M., Staskiewicz, A., 2003.
Hydrodynamic
modelling in the Polish zone of the Baltic Sea – an overview of Polish
achievements. Oceanologia 45(1), 107—120.
https://doi.org/10.1016/S0078-3234(03)00053-0
Kruk-Dowgiałło, L., Szaniawska, A., 2008.
Gulf of Gdańsk and Puck Bay.
[in:] Schiewer, U. (ed.), Ecology of Baltic Coastal Waters. Ecological
Studies, Berlin, Heidelberg, 139—165.
https://doi.org/10.1007/978-3-540-73524-3_7
Lehmann, A., Javidpour, J., 2010.
Potential pathways of invasion and
dispersal of Mnemiopsis leidyi A. Agassiz 1865 in the Baltic Sea.
Hydrobiologia 649, 107—114.
https://doi.org/10.1007/s10750-010-0233-8
Lehtiniemi, M., Gorokhova, E., Bolte, S., Haslob, H., Huwer, B., Katajisto, T.,
Lennuk, L., Markkula, S., Põllumäe, A., Schaber, M., Setälä, O.,
Reusch, T.B.H., Viitasalo-Frösén, S., Vuorinen, I., Välipakka, P., 2013.
Distribution and reproduction of the Arctic ctenophore Mertensia ovum in
the Baltic Sea. Mar. Ecol. Prog. Ser. 491, 111—124.
https://doi.org/10.3354/meps10464
Leppäkoski, E., Gollasch, S., Gruszka, P., Ojaveer, H., Olenin, S., Panov,
V., 2002.
The Baltic—a sea of invaders. Can. J. Fish. Aquat. Sci.
59, 1175—1188.
https://doi.org/10.1139/f02-089
Liblik, T., Väli, G., Salm, K., Laanemets, J., Lilover, M.-J., Lips, U.,
2022.
Quasi-steady circulation regimes in the Baltic Sea. Ocean Sci.
18 (4), 857—879.
https://doi.org/10.5194/os-18-857-2022
McKenzie, C., Behrens, J., Blakeslee, A., Canning-Clode, J., Chainho, P., Copp,
G.H., Curd, A., Darling, J., Davison, P., Galil, B., Gislason, S., Gollasch,
S., Hegele-Drywa, J., Heibeck, N., Howland, K., Jaspers, C., Jelmert, A.,
Jensen, K.R., Kakkonen, J., Kerckhof, F., Lehtiniemi, M., Marchini, A.,
Naddafi, R., Normant-Saremba, M., Occhipinti-Ambrogi, A., Olenin, S., Celmente,
R.C., Simard, N., Smolders, S., Viard, F., Zabrocki, M., Zenetos, A., 2022.
Working Group on Introductions and Transfers of Marine Organisms
(WGITMO). International Council for the Exploration of the Sea (ICES).
ICES Sci.Rep. 4 (84), 216 pp.
Myrberg, K., Lehmann, A., 2013.
Topography, Hydrography, Circulation and
Modelling of the Baltic Sea. [in:] Soomere, T., Quak, E. (eds.),
Preventive Methods for Coastal Protection. Springer, Heidelberg, 31—64.
https://doi.org/10.1007/978-3-319-00440-2_2
Nogueira Jr., M., de Oliveira, J.S., 2006.
Moerisia inkermanica
Paltschikowa-Ostroumova (Hydrozoa; Moerisidae) e Blackfordia virginica Mayer
(Hydrozoa; Blackfordiidae) na Baía de Antonina, Paraná, Brasil. Pan-Am.
J. Aquat. Sci. 1 (1), 35—42.
https://doi.org/10.1111/maec.12119
Olenycz, M., 2015.
Gelatinous zooplankton – a potential threat to the
ecosystem of the Puck Bay (the southern Baltic Sea, Poland). Bull,
Maritime Inst. Gdańsk 30 (1), 78—85.
https://doi.org/10.5604/12307424.1172820
Restaino, D.J., Bologna, P.A., Gaynor, J.J., Buchanan, G.A., Bilinski, J.J.,
2018.
Who’s lurking in your lagoon? First occurrence of the invasive
hydrozoan Moerisia sp. (Cnidaria: Hydrozoa) in New Jersey, USA.
BioInvasions Rec. 7 (3), 223—228.
https://doi.org/10.3391/bir.2018.7.3.02
Saraber, J.G.A.M., 1962.
Ostroumovia inkermanica in the Netherlands.
Beaufortia 9, 117—120.
Schuchert, P., 2010.
The European athecate hydroids and their medusae
(Hydrozoa, Cnidaria): Capitata Part 2. Rev. Suisse Zool. 117 (3),
337—555.
https://doi.org/10.5962/bhl.part.117793
Wintzer, A.P., Meek, M.H., Moyle, P.B., 2011.
Life history and population
dynamics of Moerisia sp., a non-native hydrozoan, in the upper San Francisco
Estuary (U.S.A.). Estuarine, Estuar. Coast. Shelf Sci. 94, 48—55.
https://doi.org/10.1016/j.ecss.2011.05.017
Does mesh size matter? Influence of mesh size on estimation of meiofauna abundance, biomass and on size spectra
Oceanologia, 67 (4)/2025, 67411, 10 pp.
https://doi.org/10.5697/RXWV1889
Barbara Górska1,*, Katarzyna Grzelak1, Bodil A. Bluhm2, Silvia Hess3, Maria Włodarska-Kowalczuk1
1Institute of Oceanology Polish Academy of Sciences, ul. Powstańców Warszawy 55, 81–712 Sopot, Poland;
e-mail: basia@iopan.pl (B. Górska)
2UiT The Arctic University of Norway, PO Box 6050 Stakkevollan, N–9037 Tromsø, Norway
3University of Oslo, PO Box 1047 Blindern, NO–0316 Oslo, Norway
*corresponding author
Keywords:
Meiofauna; Biodiversity; Processing methods; Size spectra
Received: 29 May 2025; revised: 22 October 2025; accepted: 28 October 2025
Highlights
- 8-21% of individuals passed through the 63µm mesh and were retained on the 32µm sieve.
- Biomass was barely affected by the use of the coarser mesh size.
- No significant difference in abundance/biomass size spectra was observed between sieve sizes.
Abstract
For muddy-bottom meiofauna analyses, samples are typically sieved on 32 µm sieves for animal extraction. However, some studies use a 63 µm sieve to reduce fine sediment overload. Such variation in sieving protocols hampers comparisons across studies. We quantified and compared the effects of mesh size (63 versus 32 µm) on meiofauna
abundance, biomass, community composition, and size spectra in muddy sediments. Between 8 and 21% of individuals (mostly nematodes) were missed in the 63 µm mesh-based analyses (compared to 32 µm). However, the larger mesh size did not affect biomass estimations or the construction of size spectra. For muddy sediments, a 63 µm sieve can be used interchangeably with a 32 µm sieve for meiofauna biomass estimation.
References 
Aller, R.C., Aller, J.Y., 1992.
Meiofauna and solute transport in marine
muds. Limnol. Oceanogr. 37, 1018–1033.
https://doi.org/10.4319/lo.1992.37.5.1018
Anderson, M.J., 2005.
PERMANOVA: a FORTRAN computer program for
permutational multivariate analysis of variance. Department of Statistics,
University of Auckland, New Zeland.
Baguley, J.G., Hyde, L.J., Montagna, P.A., 2004.
A semi-automated digital
microphotographic approach to measure meiofaunal biomass. Limnol.
Oceanogr. Methods 2, 181–190.
https://doi.org/10.4319/lom.2004.2.181
Berkenbusch, K., Probert, P.K., Nodder, S.D., 2011.
Comparative biomass of
sediment benthos across a depth transect, Chatham Rise, Southwest Pacific
Ocean. Mar. Ecol. Prog. Ser. 425, 79–90.
https://doi.org/10.3354/meps09014
Bessière, A., Nozais, C., Brugel, S., Demers, S., Desrosiers, G., 2007.
Metazoan meiofauna dynamics and pelagicbenthic coupling in the Southeastern
Beaufort Sea, Arctic Ocean. Polar Biol. 30, 1123–1135.
https://doi.org/10.1007/s00300-007-0270-6
Bonaglia, S., Nascimento, F.J.A., Bartoli, M., Klawonn, I., Brüchert, V.,
2014.
Meiofauna increases bacterial denitrification in marine
sediments. Nat. Commun. 5, 5133.
https://doi.org/10.1038/ncomms6133
Brown, C.J., Lambshead, P.J.D., Smith, C.R., Hawkins, L.E., Farley, R., 2001.
Phytodetritus and the abundance and biomass of abyssal nematodes in the
central, equatorial Pacific. Deep Sea Res. Pt. I, 48, 555–565.
https://doi.org/10.1016/S0967-0637(00)00049-2
Charrier, B.R., Ingels, J., Danielson, S.L., Mincks, S.L., 2023.
Infaunal
community structure, diversity, and function in Pacific-Arctic shelf sediments:
a comparison of meiofaunaland macrofaunal-sized nematodes. Mar. Ecol.
Prog. Ser. 720, 95–116.
https://doi.org/10.3354/meps14397
Danovaro, R., Della Croce, N., Eleftheriou, A., Fabiano, M., Papadopoulou, N.,
Smith, C., Tselepides, A., 1995.
Meiofauna of the deep Eastern
Mediterranean Sea: distribution and abundance in relation to bacterial biomass,
organic matter composition and other environmental factors. Prog.
Oceanogr. 36, 329–341.
Danovaro, R., Gambi, C., Della Croce, N., 2002.
Meiofauna hotspot in the
Atacama Trench, eastern South Pacific Ocean. Deep Sea Res. Pt. I, 49,
843–857.
https://doi.org/10.1016/S0967-0637(01)00084-X
de Bovée, F., Soyer, J., Albert, P., 1974.
The importance of the mesh
size for the extraction of the muddy bottom meiofauna. Limnol. Oceanogr.
19, 350–354.
https://doi.org/10.4319/lo.1974.19.2.0350
Elmgren, R., 1973.
Methods of sampling sublittoral soft bottom
meiofauna. OIKOS Suppl. 112–120.
Feller, R.J., Warwick, R.M., 1988.
Energetics, [In:] Introduction to the
Study of Meiofauna, Higgins, R.P., Thiel, H. (Eds.), Smithsonian Inst.
Press, Washington D.C., 181–196.
Giere, O., 2009. Meiobenthology: the microscopic motile fauna of aquatic
sediments. Springer, Berlin.
Gooday, A.J., Pfannkuche, O., Lambshead, P.J.D., 1996.
An apparent lack of
response by metazoan meiofauna to phytodetritus deposition in the bathyal
north-eastern Atlantic. J. Mar. Biol. Assoc. U.K. 76, 297–310.
Górska, B., Soltwedel, T., Schewe, I., Włodarska-Kowalczuk, M., 2020.
Bathymetric trends in biomass size spectra, carbon demand, and production
of Arctic benthos (76-5561 m, Fram Strait). Prog. Oceanogr. 186, 102370.
https://doi.org/10.1016/j.pocean.2020.102370
Górska, B., Włodarska-Kowalczuk, M., 2017.
Food and disturbance effects
on Arctic benthic biomass and production size spectra. Prog. Oceanogr.
152, 50–61.
https://doi.org/10.1016/j.pocean.2017.02.005
Grove, S.L., Probert, P.K., Berkenbusch, K., Nodder, S.D., 2006.
Distribution of bathyal meiofauna in the region of the Subtropical Front,
Chatham Rise, south-west Pacific. J. Exp. Mar. Biol. Ecol. 330, 342–355.
https://doi.org/10.1016/j.jembe.2005.12.038
Grzelak, K., Gluchowska, M., Kędra, M., Błażewicz, M., 2020.
Nematode
responses to an Arctic sea-ice regime: morphometric characteristics and biomass
size spectra. Mar. Environ. Res. 162.
https://doi.org/10.1016/j.marenvres.2020.105181
Hughes, D.J., Gage, J.D., 2004.
Benthic metazoan biomass, community
structure and bioturbation at three contrasting deep-water sites on the
northwest European continental margin. Prog. Oceanogr. 63, 29–55.
https://doi.org/10.1016/j.pocean.2004.09.002
Ingole, B.S., Goltekar, R., Gonsalves, S., Ansari, Z.A., 2005.
Recovery of
deep-sea meiofauna after artificial disturbance in the central indian
basin. Mar. Georesour. Geotec. 23, 253–266.
https://doi.org/10.1080/10641190500446540
Kitahashi, T., Watanabe, H.K., Tsuchiya, M., Yamamoto, Hideyuki, Yamamoto,
Hiroyuki, 2018.
A new method for acquiring images of meiobenthic images
using the FlowCAM. MethodsX 5, 1330–1335.
https://doi.org/10.1016/j.mex.2018.10.012
Kotwicki, L., Szymelfenig, M., De Troch, M., Zajączkowski, M., 2004.
Distribution of meiofauna in Kongsfjorden, Spitsbergen. Polar Biol.
27, 661–669.
https://doi.org/10.1007/s00300-004-0625-1
Lampadariou, N., Eleftheriou, A., 2018.
Seasonal dynamics of meiofauna from
the oligotrophic continental shelf of Crete (Aegean Sea, eastern
Mediterranean). J. Exp. Mar. Biol. Ecol. 502, 91–104.
https://doi.org/10.1016/j.jembe.2017.12.014
Leduc, D., Probert, P.K., Nodder, S.D., 2010.
Influence of mesh size and
core penetration on estimates of deepsea nematode abundance, biomass, and
diversity. Deep Sea Res. Pt. I, 57, 1354–1362.
https://doi.org/10.1016/j.dsr.2010.06.005
Mazurkiewicz, M., Górska, B., Jankowska, E., Włodarska-Kowalczuk, M., 2016.
Assessment of nematode biomass in marine sediments: A semi-automated image
analysis method. Limnol. Oceanogr. Methods 14, 816–827.
https://doi.org/10.1002/lom3.10128
Nascimento, F.J.A., Karlson, A.M.L., Näslund, J., Elmgren, R., 2011.
Diversity of larger consumers enhances interference competition effects on
smaller competitors. Oecologia 166, 337–347.
https://doi.org/10.1007/s00442-010-1865-0
Peters, R., 1983.
The ecological implications of body size. Cambridge
Univ. Press, Cambridge.
Ricardo de Freitas, T., Hess, S., Renaud, P.E., Appleby, P., Alve, E., 2024.
Drivers of organic carbon distribution and accumulation in the northern
Barents Sea. Prog. Oceanogr. 225, 103286.
https://doi.org/10.1016/j.pocean.2024.103286
Sautya, S., Gaikwad, S., Khokher, S., Pradhan, U.K., Chatterjee, S., Choudhury,
A., Sahu, B., Attri, S., 2021.
Distribution Pattern of the Benthic
Meiofaunal Community Along the Depth Gradient of the Western Indian Continental
Margin, Including the OMZ and Abyssal Plain. Front. Mar. Sci. 8, 1–17.
https://doi.org/10.3389/fmars.2021.671444
Schewe, I., Soltwedel, T., 1999.
Deep-sea meiobenthos of the central Arctic
Ocean: distribution patterns and sizestructure under extreme oligotrophic
conditions. Vie Milieu 49, 79–92.
Schwinghamer, P., 1983.
Generating ecological hypotheses from biomass
spectra using causal analysis: a benthic example. Mar. Ecol. Prog. Ser.
13, 151–166.
Sheldon, R.W., Prakash, A., Sutcliffe, H., 1972.
The size distribution of
particles in the Ocean. Limnol. Oceanogr. 17, 327–340.
Soltwedel, T., Miljutina, M., Mokievsky, V., Thistle, D., Vopel, K., 2003.
The meiobenthos of the Molloy Deep (5 600 m), Fram Strait, Arctic
Ocean. Vie Milieu 53, 1–13.
Tachibana, K., Shimanaga, M., Langlet, D., Seike, K., Miyazaki, M., Yoshida,
M., Nunoura, T., Nomaki, H., 2023.
Meiofauna in the southeastern Bering
Sea: community composition and structuring environmental factors. Front.
Mar. Sci. 10, 1–11.
https://doi.org/10.3389/fmars.2023.996380
The Nansen Legacy, 2025.
The Nansen Legacy Final Report. Nansen Leg.
Rep. Ser.
https://doi.org/10.7557/nlrs.8041
Tietjen, J.H., 1992.
Abundance and biomass of metazoan meiobenthos in the
deep sea. [In:] Deep-Sea Food Chains and the Global Carbon Cycle, Rowe,
G.T., Pariente, V. (Eds.), Springer, College Station, 45–62.
https://doi.org/10.1007/978-94-011-2452-2_4
Tong, S.J.W., Gan, B.Q., Tan, K.S., 2022.
Community structure of deep-sea
benthic metazoan meiofauna in the polymetallic nodule fields in the eastern
Clarion-Clipperton Fracture Zone, Pacific Ocean. Deep Sea Res. Pt. I, 188.
https://doi.org/10.1016/j.dsr.2022.103847
Tseitlin, V.B., Mokievsky, V.O., Azovsky, A.I., Soltwedel, T., 2001.
The
study of meiobenthos size structure with the sieving method (case study of the
Arctic basin free-living Nematodes). Oceanology 41, 712–717.
Udalov, A.A., Azovsky, A.I., Mokievsky, V.O., 2005.
Depthrelated pattern in
nematode size: What does the depth itself really mean? Prog. Oceanogr. 67,
1–23.
https://doi.org/10.1016/j.pocean.2005.02.020
Vanreusel, A., Clough, L., Jacobsen, K., Ambrose, W., Ryheul, V., Herman, R.,
Vincx, M., 2000.
Meiobenthos of the central Arctic Ocean with special
emphasis on the nematode community structure. Deep Sea Res. Pt. I, 47,
1855–1879.
https://doi.org/10.1016/S0967-0637(00)00007-8
Vanreusel, A., Vincx, M., Bett, B.J., Rice, A.L., 1995.
Nematode biomass
spectra at two abyssal sites in the NE Atlantic with a contrasting food
supply. Int. Rev. Hydrobiol. 80, 287–296.
Zeng, Q., Huang, D., Lin, R., Wang, J., 2017.
Deep-sea metazoan meiofauna
from a polymetallic nodule area in the central Indian Ocean basin. Mar.
Biodivers. 48, 395–405.
https://doi.org/10.1007/s12526-017-0778-0
Zeppilli, D., Sarrazin, J., Leduc, D., Arbizu, P.M., Fontaneto, D., Fontanier,
C., Gooday, A.J., Kristensen, R.M., Ivanenko, V.N., Sørensen, M. V.,
Vanreusel, A., Thébault, J., Mea, M., Allio, N., Andro, T., Arvigo, A.,
Castrec, J., Danielo, M., Foulon, V., Fumeron, R., Hermabessiere, L., Hulot,
V., James, T., Langonne-Augen, R., Le Bot, T., Long, M., Mahabror, D., Morel,
Q., Pantalos, M., Pouplard, E., Raimondeau, L., Rio-Cabello, A., Seite, S.,
Traisnel, G., Urvoy, K., Van Der Stegen, T., Weyand, M., Fernandes, D., 2015.
Is the meiofauna a good indicator for climate change and anthropogenic
impacts? Mar. Biodivers. 45, 505–535.
https://doi.org/10.1007/s12526-015-0359-z












